-
PDF
- Split View
-
Views
-
Cite
Cite
D.A. Goldstein, J.A. Thomas, Biopharmaceuticals derived from genetically modified plants, QJM: An International Journal of Medicine, Volume 97, Issue 11, November 2004, Pages 705–716, https://doi.org/10.1093/qjmed/hch121
Close - Share Icon Share
Abstract
Modern biotechnology has resulted in a resurgence of interest in the production of new therapeutic agents using botanical sources. With nearly 500 biotechnology products approved or in development globally, and with production capacity limited, the need for efficient means of therapeutic protein production is apparent. Through genetic engineering, plants can now be used to produce pharmacologically active proteins, including mammalian antibodies, blood product substitutes, vaccines, hormones, cytokines, and a variety of other therapeutic agents. Efficient biopharmaceutical production in plants involves the proper selection of host plant and gene expression system, including a decision as to whether a food crop or a non-food crop is more appropriate. Product safety issues relevant to patients, pharmaceutical workers, and the general public must be addressed, and proper regulation and regulatory oversight must be in place prior to commercial plant-based biopharmaceutical production. Plant production of pharmaceuticals holds great potential, and may become an important production system for a variety of new biopharmaceutical products.
Introduction
The use of plants or their extracts for the treatment of human disease predates the earliest stages of recorded civilization, dating back at least to the Neanderthal period. By the 16th century, botanical gardens provided a wealth of materia medica for teaching therapeutic use; and herbal medicine flourished until the 17th century when more scientific ‘pharmacological’ remedies were discovered.1 Subsequently, the active principle in many medicinal plants was identified and, in many cases, purified for therapeutic use. Even today, about one-fourth of current prescription drugs have a botanical origin.1
Modern biotechnology has led to a resurgence of interest in obtaining new medicinal agents from botanical sources. Through genetic engineering (GE), plants can now be used to produce a variety of proteins, including mammalian antibodies, blood substitutes, vaccines and other therapeutic entities.2 Recently, the production of foreign proteins in GE plants has become a viable alternative to conventional production systems such as microbial fermentation or mammalian cell culture. GE plants, acting as bioreactors, can efficiently produce recombinant proteins in larger quantities than those produced using mammalian cell systems.3 Plant-derived proteins are particularly attractive, since they are free of human diseases and mammalian viral vectors. Large quantities of biomass can be easily grown in the field, and may permit storage of material prior to processing. Thus, plants offer the potential for efficient, large-scale production of recombinant proteins with increased freedom from contaminating human pathogens.
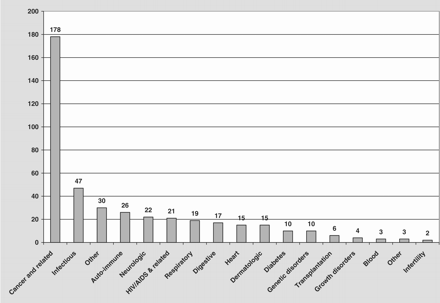
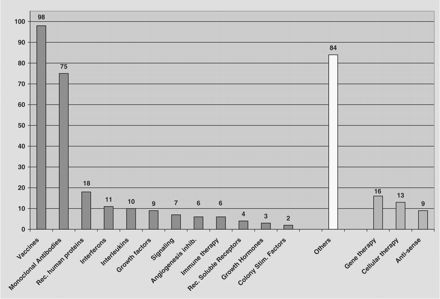
During the last two decades, approximately 95 biopharmaceutical products have been approved by one or more regulatory agencies for the treatment of various human diseases including diabetes mellitus, growth disorders, neurological and genetic maladies, inflammatory conditions, and blood dyscrasias.4–6 Some 500 agents are believed to be in development world-wide, with some 370 biopharmaceuticals in the US, including 178 agents directed against cancer or related conditions, 47 against infectious diseases, and the remainder for a variety of important medical conditions (Figure 1).6 Among these therapeutic entities are recombinant proteins, monoclonal antibodies, antisense oligonucleotides, and a variety of other protein agents such as hormones and immunomodulating drugs (Figure 2). This rapid increase in the number of new protein and peptide drugs reflects rapid advances in molecular biology, highlighted by the success of the human genome project that, in turn, will help to identify many additional opportunities for therapeutic intervention. Unfortunately, our capacity to produce these proteins in the quantities needed is expected to fall far short of demand by the end of the current decade.7 While none of the commercially available products are currently produced in plants, those biotechnology products which are comprised of proteins, and possibly also DNA-based vaccines, are potential candidates for plant-based production.
Number of biopharmaceuticals under development, by disease class as of 2003.6
Number of biopharmaceuticals under development, by type of agent.6
Advances in plant biotechnology have already resulted in plants that produce monoclonal antibodies or other therapeutic proteins, or that may serve as a source of edible vaccines. Research now underway will almost certainly result in GE plants designed to produce other therapeutic agents including hormones (e.g. insulin, somatotropin, erythropoietin), blood components, coagulation factors, and various interferons, and may well avoid critical limitations in production capacity.
Transgenic pharmaceutical plants are primarily modified by the introduction of novel gene sequences which drive the production of ‘designer’ proteins or peptides. These proteins or peptides possess therapeutic value themselves, have properties that allow them to be used as precursors in the synthesis of medicinal compounds, or may serve as technical enzymes in pharmaceutical production. This review will attempt to catalogue the potential therapeutic applications of plant biotechnology and to address concerns related to the safety and efficacy of these agents in relation to human health and to specific disease states.
The why and how of plant biotechnology
Plant biotechnology can lead to the commercial production of pharmacologically important proteins which are, in many cases, fully functional and nearly identical to their mammalian counterparts.2 The application of plant biotechnology to produce hormones or other biologically active molecules began nearly 20 years ago, with a crucial advance being the expression of functional antibodies in plants, thereby demonstrating that plants could produce complex proteins of therapeutic significance.2 While bacteria are inexpensive and convenient production systems for many proteins (e.g. human insulin), they are incapable of the post-translational modification and assembly steps required for biological activity in more complex multi-component proteins such as antibodies.2 Plants exhibit an effective eukaryote protein synthesis pathway, and by combining currently available gene expression systems with appropriate acreage, plants can readily produce ton quantities of protein.2 Unlike mammalian cell systems, which can sometimes express pathogenic viral agents, plant systems are intrinsically free of mammalian pathogens.8 Thus, plant expression systems may offer advantages over bacterial and mammalian cell culture systems (Table 1).2
Comparison of recombinant protein production in plants, yeast and mammalian systems
| . | Transgenic plants . | Plant viruses . | Yeast . | Bacteria . | Mammalian cell cultures . | Transgenic animals . |
|---|---|---|---|---|---|---|
| Cost/storage | Cheap/RT | Cheap/−20°C | Cheap/−20°C | Cheap/−20°C | Expensive | Expensive |
| Distribution | Easy | Easy | Feasible | Feasible | Difficult | Difficult |
| Gene size | Not limited | Limited | Unknown | Unknown | Limited | Limited |
| Glycosylation | ‘Correct'? | ‘Correct'? | Incorrect | Absent | ‘Correct’ | ‘Correct’ |
| Multimeric protein assembly (SigA) | Yes | No | No | No | No | Yes |
| Production cost | Low | Low | Medium | Medium | High | High |
| Production scale | Worldwide | Worldwide | Limited | Limited | Limited | Limited |
| Production vehicle | Yes | Yes | Yes | Yes | Yes | Yes |
| Propagation | Easy | Feasible | Easy | Easy | Hard | Feasible |
| Protein folding accuracy | High? | High? | Medium | Low | High | High |
| Protein homogeneity | High? | Medium | Medium | Low | Medium | High |
| Protein yield | High | Very high | High | Medium | Medium-High | High |
| Public perception of risk | High | High | Medium | Low | Medium | High |
| Safety | High | High | Unknown | Low | Medium | High |
| Scale-up costs | Low | Low | High** | High** | High** | High |
| Therapeutic risk* | Unknown | Unknown | Unknown | Yes | Yes | Yes |
| Time required | Medium | Low | Medium | Low | High | High |
| . | Transgenic plants . | Plant viruses . | Yeast . | Bacteria . | Mammalian cell cultures . | Transgenic animals . |
|---|---|---|---|---|---|---|
| Cost/storage | Cheap/RT | Cheap/−20°C | Cheap/−20°C | Cheap/−20°C | Expensive | Expensive |
| Distribution | Easy | Easy | Feasible | Feasible | Difficult | Difficult |
| Gene size | Not limited | Limited | Unknown | Unknown | Limited | Limited |
| Glycosylation | ‘Correct'? | ‘Correct'? | Incorrect | Absent | ‘Correct’ | ‘Correct’ |
| Multimeric protein assembly (SigA) | Yes | No | No | No | No | Yes |
| Production cost | Low | Low | Medium | Medium | High | High |
| Production scale | Worldwide | Worldwide | Limited | Limited | Limited | Limited |
| Production vehicle | Yes | Yes | Yes | Yes | Yes | Yes |
| Propagation | Easy | Feasible | Easy | Easy | Hard | Feasible |
| Protein folding accuracy | High? | High? | Medium | Low | High | High |
| Protein homogeneity | High? | Medium | Medium | Low | Medium | High |
| Protein yield | High | Very high | High | Medium | Medium-High | High |
| Public perception of risk | High | High | Medium | Low | Medium | High |
| Safety | High | High | Unknown | Low | Medium | High |
| Scale-up costs | Low | Low | High** | High** | High** | High |
| Therapeutic risk* | Unknown | Unknown | Unknown | Yes | Yes | Yes |
| Time required | Medium | Low | Medium | Low | High | High |
*Residual viral sequences, oncogenes, endotoxins; **Large, expensive fermenters. Table modified from Fischer & Emans.2
Comparison of recombinant protein production in plants, yeast and mammalian systems
| . | Transgenic plants . | Plant viruses . | Yeast . | Bacteria . | Mammalian cell cultures . | Transgenic animals . |
|---|---|---|---|---|---|---|
| Cost/storage | Cheap/RT | Cheap/−20°C | Cheap/−20°C | Cheap/−20°C | Expensive | Expensive |
| Distribution | Easy | Easy | Feasible | Feasible | Difficult | Difficult |
| Gene size | Not limited | Limited | Unknown | Unknown | Limited | Limited |
| Glycosylation | ‘Correct'? | ‘Correct'? | Incorrect | Absent | ‘Correct’ | ‘Correct’ |
| Multimeric protein assembly (SigA) | Yes | No | No | No | No | Yes |
| Production cost | Low | Low | Medium | Medium | High | High |
| Production scale | Worldwide | Worldwide | Limited | Limited | Limited | Limited |
| Production vehicle | Yes | Yes | Yes | Yes | Yes | Yes |
| Propagation | Easy | Feasible | Easy | Easy | Hard | Feasible |
| Protein folding accuracy | High? | High? | Medium | Low | High | High |
| Protein homogeneity | High? | Medium | Medium | Low | Medium | High |
| Protein yield | High | Very high | High | Medium | Medium-High | High |
| Public perception of risk | High | High | Medium | Low | Medium | High |
| Safety | High | High | Unknown | Low | Medium | High |
| Scale-up costs | Low | Low | High** | High** | High** | High |
| Therapeutic risk* | Unknown | Unknown | Unknown | Yes | Yes | Yes |
| Time required | Medium | Low | Medium | Low | High | High |
| . | Transgenic plants . | Plant viruses . | Yeast . | Bacteria . | Mammalian cell cultures . | Transgenic animals . |
|---|---|---|---|---|---|---|
| Cost/storage | Cheap/RT | Cheap/−20°C | Cheap/−20°C | Cheap/−20°C | Expensive | Expensive |
| Distribution | Easy | Easy | Feasible | Feasible | Difficult | Difficult |
| Gene size | Not limited | Limited | Unknown | Unknown | Limited | Limited |
| Glycosylation | ‘Correct'? | ‘Correct'? | Incorrect | Absent | ‘Correct’ | ‘Correct’ |
| Multimeric protein assembly (SigA) | Yes | No | No | No | No | Yes |
| Production cost | Low | Low | Medium | Medium | High | High |
| Production scale | Worldwide | Worldwide | Limited | Limited | Limited | Limited |
| Production vehicle | Yes | Yes | Yes | Yes | Yes | Yes |
| Propagation | Easy | Feasible | Easy | Easy | Hard | Feasible |
| Protein folding accuracy | High? | High? | Medium | Low | High | High |
| Protein homogeneity | High? | Medium | Medium | Low | Medium | High |
| Protein yield | High | Very high | High | Medium | Medium-High | High |
| Public perception of risk | High | High | Medium | Low | Medium | High |
| Safety | High | High | Unknown | Low | Medium | High |
| Scale-up costs | Low | Low | High** | High** | High** | High |
| Therapeutic risk* | Unknown | Unknown | Unknown | Yes | Yes | Yes |
| Time required | Medium | Low | Medium | Low | High | High |
*Residual viral sequences, oncogenes, endotoxins; **Large, expensive fermenters. Table modified from Fischer & Emans.2
Biopharmaceutical production in plants necessitates a series of careful decisions regarding three critical areas: (i) the gene expression system to be used, (ii) the location of gene expression within the plant, and (iii) the type of plant to be used.
There are a number of gene expression strategies that can be used to produce specific proteins in plants. With transient expression (TE), a gene sequence is inserted into plant cells using plant viruses, ballistic (gene-gun), or other methods, without incorporation of the new genetic material into the plant chromosome. TE systems can be rapidly deployed and can produce large amounts of protein,2 but because non-chromosomal DNA is not copied with the process of mitosis or meiosis, gene expression is neither permanent nor heritable. While TE systems are very useful for research and development, and may be useful for drug production, they require the fresh production of transformed plants with each planting and may be less attractive for long-term or high-volume protein production.
Alternatively, the primary plant chromosome can be altered to allow for the permanent and heritable expression of a particular protein, i.e. allow the creation of plants which produce seed carrying the desired modification. This can be done using Agrobacterium tumefaciens, a pathogen of plants that, in nature, transfers genetic material to the plant chromosome. By modifying the genetic content of Agrobacterium, desired genes can be readily inserted into many kinds of plants, especially dicots such as soybean.2,9 Genetic material can also be coated onto small metallic pellets and introduced into cells ballistically using a ‘gene-gun’.9 This latter system is useful for a wide variety of plant species. While permanent modification of the plant genome is more costly and time-consuming, it offers the clear advantage of stable, ongoing protein production with repeated planting alone.
Finally, systems exist that modify chloroplast DNA in plants and that can lead to heritable changes in protein expression.3 Plant chloroplasts may play a critical role in the future development of biopharmaceuticals. These tiny energy-producing organelles appear to possess advantages over nuclear transformation, particularly given that each cell may carry hundreds or thousands of such organelles, resulting in the ability to sustain very high numbers of functional gene copies. Transgenic tobacco chloroplasts, for example, can produce human somatotropin at protein levels over a hundred-fold higher than do their nuclear transgenic counterparts, with production of somatotropin and Bt insecticidal protein representing 7% and 45% of total plant protein production.10 In the final analyses, the selection of a plant expression system is influenced by cost, safety, and production factors.
Consideration must also be given to where within the plant a pharmaceutical protein is to be produced. Current technology allows gene expression and protein production in either the green matter of the plant (whole plant expression) or selectively in the seed or other tissues through the use of selective promoter systems.11 While production in green mass can produce large amounts of protein,3 green matter is highly physiologically active and protein levels may be poorly preserved if materials are not rapidly dried or otherwise inactivated.8,11 Thus, unless a protein or peptide is highly stable, green matter production may result in poor protein recovery and usually requires immediate processing. Tuber or root production, while feasible, shares many of the characteristics of green matter production systems. Unlike green matter, seeds generally contain fewer phenolic compounds and a less complex mixture of proteins and have specifically evolved to provide for stable, long-term storage of proteins and other materials in order to assure successful, delayed germination.3 Seeds are therefore an extremely attractive production medium which can also provide the flexibility to store product for delayed processing.
It is also necessary to decide which plant species to transform for production of a specific pharmaceutical product. While nearly any plant could theoretically be transformed, practical considerations suggest the use of plants with which we are most familiar, and which already have well-established techniques for genetic transformation, high volume production, harvest, and processing. For green matter production, tobacco has usually been the material of choice, largely because of its highly efficient production of biomass,2 although other systems such as alfalfa and even duckweed show promise.12 For seed production, a plant optimized for large seed and high protein production is clearly preferred. Food crop plants have been bred specifically to produce highly productive stands of high-protein seed for which harvesting, processing, and storage technologies are already available. Further, techniques for genetic modification of these plants are well understood, and the extensive history of cultivation and genetic research provides both an understanding of genetic stability and a pool of genetic resources (such as the ability to control pollination using the classical C-male-sterile gene in corn), which facilitate production. This makes food crops highly attractive, with soybean and maize (corn) being the obvious choices. This choice, while highly rational, does lead to the potential for the unintended presence of therapeutic protein in human food, and thus necessitates carefully controlled production to avoid the inadvertent presence of therapeutic material in foods, as discussed below.
Production, safety and efficacy
Drug research is a unique multi-disciplinary process leading to the development of novel therapeutic agents for disease states that have unmet needs.13 The search for new biopharmaceuticals is driven by a medical need and by the perceived likelihood of technological success, as determined by both therapeutic efficacy and safety parameters. There are several factors to consider for the safety testing of a new biopharmaceuticals.14 Because of the protein nature of most biopharmaceutical products, few non-allergic adverse reactions other than those attributable to the primary pharmacological activity are anticipated. Nevertheless, both Good Laboratory Practice and Good Manufacturing Practice, as established for other modes of pharmaceutical production, are essential to plant made pharmaceuticals. Before experimental or clinical use is initiated, it is critical to have fully-characterized, contaminant-free materials, as well as appropriate quality assurance so that both the product itself and the therapeutic results will be reproducible. New pharmaceutical agents derived through plant biotechnology must be subjected to the same purity, quality-control, and safety standards as materials derived from bacterial or mammalian cell systems or from other traditional sources such as vaccine production.
Sites used for the cultivation of genetically modified plants have in some cases been disrupted or destroyed by individuals opposed to the use of plant biotechnology, raising additional security concerns. In part, these concerns can be addressed via increased field site monitoring and security, and the use of enclosed environments (greenhouses) for small scale operations. The relatively small scale and favourable economics of biopharmaceutical operations allows the placement of field operations in geopolitical locations selected for optimal security, with subsequent shipping of raw or processed materials.
Transgenic plants have the added safety feature of freedom from human or animal pathogens.8 Additionally, plant cells are capable of producing complex proteins while largely avoiding the presence of endotoxins in bacterial systems. Endotoxins are often difficult to remove and can contaminate a final product. Thus, there is intrinsic safety and value in using plants as a source of recombinant protein.15 However, as with all plant-derived pharmaceuticals, appropriate measures must be taken to eliminate undesirable plant-derived proteins or other biomolecules and to control the presence of fungal toxins or of pesticides used in plant production.11
Safety evaluations must consider possible non-target organ responses as well as the entire gamut of anticipated and unanticipated side-effects as with any bio-pharmaceutical product. Somewhat unique to plant-produced pharmaceuticals are potential effects on non-target species such as butterflies, honeybee, and other wildlife at or near the growing sites. Fortunately, in most instances, the effect on non-target species is limited by the fact that proteins are a normal part of the diet, are readily digested, and are degraded in the environment. Further, many biopharmaceuticals proteins, especially antibodies, are highly species-specific in their effects.
Pharmaceutical production in plants may create the potential for the flow of pharmaceutical materials into the human food chain, especially when food crops are used. This could occur as a result of inadvertent cross-contamination of foodstuffs, through spontaneous growth of genetically engineered plants where they are not desired, or by virtue of pollen flow with some plants (e.g. corn), but not others (e.g. potato). While some have therefore suggested restricting pharmaceutical production to non-food crops such as tobacco, it is the food crops that present the greatest opportunities for efficient production of biopharmaceuticals and that will be most useful for the production of edible vaccines.
Because of the potential for adventitious presence in food, care must be exercised in the production of biopharmaceuticals in food crops. Fortunately, acreage requirements for pharmaceutical production are limited, with metric ton protein production being feasible with >5000 acres of corn.9 This allows for production under tightly controlled conditions which include production in areas of the country where the crop in question is not routinely grown, the use of physical isolation distances and temporal separation to prevent cross-pollination with food crops, the use of de-tasseling and/or male-sterile traits to control pollen flow, dedicated harvest and storage equipment, and controlled processing separate from all food crops. Unlike commodity crops, plant production of pharmaceuticals should be performed only under tightly controlled conditions similar to those of other pharmaceutical manufacturing; and production standards have been developed jointly by industry, USDA, FDA, and international organizations.12 These standards are enforced in the US through USDA and FDA, and compliance is further encouraged by the desire of producers to avoid potential liability and infractions. FDA required Good Manufacturing Practice necessitates extensive control of field access, harvest, and product disposition.
While production controls are necessary and appropriate, it should be kept in mind that the majority of therapeutic proteins are not anticipated to have any pharmacological activity when ingested, and are thus unlikely to present a safety issue in the event of accidental contamination of foodstuffs. For example, antibodies, insulin, growth hormone, and most other proteins produce few, if any, systemic pharmacological effects by the oral route. This does not preclude the possibility of local effects on the gastro-intestinal tract or the possibility of immunological effects, as seen in the context of oral vaccines, where such an effect is introduced by design. In fact, one plant-derived antibody directed against epithelial cellular adhesion molecules was withdrawn from clinical development as a result of gastro-intestinal side-effects believed to be due to binding to the relevant antigen, which is expressed in the GI tract.8 This is a result of the antigenic specificity of the antibody, and is not attributable to the plant-derived nature of the molecule. While a case-by-case determination of risk will be necessary when considering proteins for food crop applications, it appears that the majority of proteins would present no great hazard to the public in the event that control technologies should fail to be fully effective.
The production of pharmaceuticals in plants
There are a number of recent comprehensive review articles pertaining to production technologies used for molecular farming in plants.3,8,9,11,15 The first commercially produced biopharmaceutical, recombinant human insulin from bacteria, was produced in 1982; an event which coincides roughly with the first development of a genetically modified plant in 1984.16,17 This latter development was followed rapidly with a demonstration of the potential of plants for pharmaceutical production with plant expression of human growth hormone fusion protein,18 interferon,19 monoclonal antibodies,20 and serum albumin.21 Since that time, numerous demonstrations of pharmaceutical production in plants have occurred and are described below within three broad categories of therapeutics: antibodies, vaccines, and other therapeutics.
Antibodies
Monoclonal antibodies (mAbs) have been critical both for the development of biotechnology itself and as products for both therapeutic and diagnostic purposes. Traditional therapeutic monoclonal antibodies have been derived from mice. These proteins were readily identified by the human immune system as foreign, limiting the utility of these antibodies for therapeutic use, especially with repeated dosing.22 Even in the absence of anaphylaxis or serum sickness, the occurrence of neutralizing antibodies which inactivate the drug often precluded further therapeutic use. However, recombinant technologies have allowed murine antibodies to be replaced with partially humanized or chimeric antibodies, and now allow the production of fully human antibodies.22 The latter may be derived from mice carrying the human immunoglobulin genes or produced using yeast or other gene-expression array technologies.9,22 Recombinant technology can also be used to selectively ‘evolve’ an antibody gene to produce higher affinity binding (affinity maturation).9 Thus, compared with earlier monoclonal antibodies, current recombinant antibodies exhibit reduced immunogenicity and increased biological activity.22,23 Recently, the first fully human therapeutic monoclonal antibody has been commercialized (Humira, Adalimumab, Abbott Laboratories), and one would anticipate a low rate of neutralizing antibody development.
Currently, there are over a dozen FDA-approved mAbs, and as many as 700 therapeutic Abs may be under development.9 Plants now have potential as a virtually unlimited source of mAbs, referred to by some as ‘plantibodies’. Tobacco plants have been used extensively for antibody expression systems. However, several other plants have been used including potatoes, soybeans, alfalfa, rice and corn. Antibody formats can be full-size, Fab fragments, single-chain antibody fragments, bi-specific scFv fragments, membrane anchored scFv, or chimeric antibodies (see Table 2).2 Plant cells, unlike mammalian cell expression systems, can express recombinant secretory IgA (sIgA). sIgA is a complex multi-subunit antibody that may be useful in topical immunotherapy, and has been successfully expressed in the tobacco plant. Transgenic soybeans are capable of producing humanized antibodies against herpes simplex virus-2. GE corn reportedly is capable of producing human antibodies at yields of up to a kg per acre,9 and has been demonstrated to preserve antibody function through five years of storage under ordinary conditions.
Recombinant antibodies expressed in transgenic plants
| Year . | Format . | Antibody/Antigen . | Plant organ . | Cellular location . | Transformed species . |
|---|---|---|---|---|---|
| 1989 | IgG1 | Phosphonate ester | Leaf | ER | Tobacco |
| 1990 | IgM | NP hapten | Leaf | ER chloroplast | Tobacco |
| 1991 | VH domain | Substance P (neuropeptide) | Leaf | Intra- & extra-cellular | N. benthamiana |
| 1992 | scFv | Phytochrome | Leaf | Cytosol | Tobacco |
| 1993 | IgG1 Fab | Human creatine kinase | Leaf | Nucleolus | Tobacco |
| 1993 | scFv | Phytochrome | Leaf | Apoplast | Tobacco |
| 1993 | scFv | AMCV | Leaf | Cytosol | N. benthamiana |
| 1994 | IgG | Fungal cutinase | Root | Apoplast | Tobacco |
| 1994 | IgG1 | Streptococcus mutans adhesin | Leaf | Apoplast | Tobacco |
| 1995 | IgA/G | Streptococcus mutans adhesin | Leaf | Apoplast | Tobacco |
| 1995 | IgG | TMV | Leaf | Apoplast | Tobacco |
| 1996 | scFv | Cutinase | Leaf | ER | Tobacco |
| 1996 | IgM | RKN secretion | Leaf root | Apoplast | Tobacco |
| 1996 | scFv | BNYVV | Leaf | Apoplast | N. benthamiana |
| 1996 | scFv | Human creatine kinase | Leaf | Cytoplasm ER | Tobacco |
| 1996 | IgG1Fab | Human creatine kinase | Leaf | Apoplast | A. thaliana |
| 1997 | scFv | β-1,4-endoglucanase | Root | Chytosol | S. tuberosum |
| 1997 | scFv | Oxazolone | Leaf | ER | Tobacco |
| 1997 | scFv | Abscisic acid | Leaf | ER | Tobacco |
| 1997 | scFv | Abscisic acid | Seed | ER | Tobacco |
| 1997 | scFv-IT | CD-40 | Plant | Apoplast | Tobacco tissue culture |
| 1998 | scFv | Oxazolone | Tuber | ER | Potato |
| 1998 | Humanized IgG | HSV-2 | Plant | Secretory pathway | Soybean |
| 1998 | scFv | Dihydro-flavonol 4-reductase | Leaf | Sytosol | P. hybrida |
| 1999 | IgG | Human IgG | Plant | Apoplast | Alfalfa |
| 1999 | scFv | CEA | Leaf | Transient expression | Tobacco |
| 1999 | scFv | Topoviruses | Plant | ER, apoplast | N. benthamiana |
| 1999 | bi-scFv | TMV | Leaf | ER, apoplast | Tobacco cell susp. |
| 1999 | scFv | TMV | Plant | Cytosol | Tobacco |
| 1999 | scFv | CEA | Cell | Er, apoplast | Rice suspension cells |
| 1999 | scFv | 38C13 mouse B-cell lymphoma | Leaf | Apolost | N. benthamiana |
| 2000 | scFv | CEA | Plant | ER, apoloast | Rice, Wheat |
| 2000 | scFv | TMV | Leaf | Apoplast, membrin | Tobacco |
| Year . | Format . | Antibody/Antigen . | Plant organ . | Cellular location . | Transformed species . |
|---|---|---|---|---|---|
| 1989 | IgG1 | Phosphonate ester | Leaf | ER | Tobacco |
| 1990 | IgM | NP hapten | Leaf | ER chloroplast | Tobacco |
| 1991 | VH domain | Substance P (neuropeptide) | Leaf | Intra- & extra-cellular | N. benthamiana |
| 1992 | scFv | Phytochrome | Leaf | Cytosol | Tobacco |
| 1993 | IgG1 Fab | Human creatine kinase | Leaf | Nucleolus | Tobacco |
| 1993 | scFv | Phytochrome | Leaf | Apoplast | Tobacco |
| 1993 | scFv | AMCV | Leaf | Cytosol | N. benthamiana |
| 1994 | IgG | Fungal cutinase | Root | Apoplast | Tobacco |
| 1994 | IgG1 | Streptococcus mutans adhesin | Leaf | Apoplast | Tobacco |
| 1995 | IgA/G | Streptococcus mutans adhesin | Leaf | Apoplast | Tobacco |
| 1995 | IgG | TMV | Leaf | Apoplast | Tobacco |
| 1996 | scFv | Cutinase | Leaf | ER | Tobacco |
| 1996 | IgM | RKN secretion | Leaf root | Apoplast | Tobacco |
| 1996 | scFv | BNYVV | Leaf | Apoplast | N. benthamiana |
| 1996 | scFv | Human creatine kinase | Leaf | Cytoplasm ER | Tobacco |
| 1996 | IgG1Fab | Human creatine kinase | Leaf | Apoplast | A. thaliana |
| 1997 | scFv | β-1,4-endoglucanase | Root | Chytosol | S. tuberosum |
| 1997 | scFv | Oxazolone | Leaf | ER | Tobacco |
| 1997 | scFv | Abscisic acid | Leaf | ER | Tobacco |
| 1997 | scFv | Abscisic acid | Seed | ER | Tobacco |
| 1997 | scFv-IT | CD-40 | Plant | Apoplast | Tobacco tissue culture |
| 1998 | scFv | Oxazolone | Tuber | ER | Potato |
| 1998 | Humanized IgG | HSV-2 | Plant | Secretory pathway | Soybean |
| 1998 | scFv | Dihydro-flavonol 4-reductase | Leaf | Sytosol | P. hybrida |
| 1999 | IgG | Human IgG | Plant | Apoplast | Alfalfa |
| 1999 | scFv | CEA | Leaf | Transient expression | Tobacco |
| 1999 | scFv | Topoviruses | Plant | ER, apoplast | N. benthamiana |
| 1999 | bi-scFv | TMV | Leaf | ER, apoplast | Tobacco cell susp. |
| 1999 | scFv | TMV | Plant | Cytosol | Tobacco |
| 1999 | scFv | CEA | Cell | Er, apoplast | Rice suspension cells |
| 1999 | scFv | 38C13 mouse B-cell lymphoma | Leaf | Apolost | N. benthamiana |
| 2000 | scFv | CEA | Plant | ER, apoloast | Rice, Wheat |
| 2000 | scFv | TMV | Leaf | Apoplast, membrin | Tobacco |
CEA, carcinoembryonic antigen; ER, endoplasmic reticulum; AMCV, artichoke mottle crinkle virus; TMV, tobacco mosaic virus; RKN, root knot nematode; BNYVV, beet necrotic yellow vein virus; HSV-2, herpes simplex virus-2; scFv-IT, scFv-bryodin-immunotoxin. N. benthamiana, tobacco (Nicotiana)-related species, A. thaliana, Arabidopsis, an experimental species. Table modified from Fischer & Emans.2
Recombinant antibodies expressed in transgenic plants
| Year . | Format . | Antibody/Antigen . | Plant organ . | Cellular location . | Transformed species . |
|---|---|---|---|---|---|
| 1989 | IgG1 | Phosphonate ester | Leaf | ER | Tobacco |
| 1990 | IgM | NP hapten | Leaf | ER chloroplast | Tobacco |
| 1991 | VH domain | Substance P (neuropeptide) | Leaf | Intra- & extra-cellular | N. benthamiana |
| 1992 | scFv | Phytochrome | Leaf | Cytosol | Tobacco |
| 1993 | IgG1 Fab | Human creatine kinase | Leaf | Nucleolus | Tobacco |
| 1993 | scFv | Phytochrome | Leaf | Apoplast | Tobacco |
| 1993 | scFv | AMCV | Leaf | Cytosol | N. benthamiana |
| 1994 | IgG | Fungal cutinase | Root | Apoplast | Tobacco |
| 1994 | IgG1 | Streptococcus mutans adhesin | Leaf | Apoplast | Tobacco |
| 1995 | IgA/G | Streptococcus mutans adhesin | Leaf | Apoplast | Tobacco |
| 1995 | IgG | TMV | Leaf | Apoplast | Tobacco |
| 1996 | scFv | Cutinase | Leaf | ER | Tobacco |
| 1996 | IgM | RKN secretion | Leaf root | Apoplast | Tobacco |
| 1996 | scFv | BNYVV | Leaf | Apoplast | N. benthamiana |
| 1996 | scFv | Human creatine kinase | Leaf | Cytoplasm ER | Tobacco |
| 1996 | IgG1Fab | Human creatine kinase | Leaf | Apoplast | A. thaliana |
| 1997 | scFv | β-1,4-endoglucanase | Root | Chytosol | S. tuberosum |
| 1997 | scFv | Oxazolone | Leaf | ER | Tobacco |
| 1997 | scFv | Abscisic acid | Leaf | ER | Tobacco |
| 1997 | scFv | Abscisic acid | Seed | ER | Tobacco |
| 1997 | scFv-IT | CD-40 | Plant | Apoplast | Tobacco tissue culture |
| 1998 | scFv | Oxazolone | Tuber | ER | Potato |
| 1998 | Humanized IgG | HSV-2 | Plant | Secretory pathway | Soybean |
| 1998 | scFv | Dihydro-flavonol 4-reductase | Leaf | Sytosol | P. hybrida |
| 1999 | IgG | Human IgG | Plant | Apoplast | Alfalfa |
| 1999 | scFv | CEA | Leaf | Transient expression | Tobacco |
| 1999 | scFv | Topoviruses | Plant | ER, apoplast | N. benthamiana |
| 1999 | bi-scFv | TMV | Leaf | ER, apoplast | Tobacco cell susp. |
| 1999 | scFv | TMV | Plant | Cytosol | Tobacco |
| 1999 | scFv | CEA | Cell | Er, apoplast | Rice suspension cells |
| 1999 | scFv | 38C13 mouse B-cell lymphoma | Leaf | Apolost | N. benthamiana |
| 2000 | scFv | CEA | Plant | ER, apoloast | Rice, Wheat |
| 2000 | scFv | TMV | Leaf | Apoplast, membrin | Tobacco |
| Year . | Format . | Antibody/Antigen . | Plant organ . | Cellular location . | Transformed species . |
|---|---|---|---|---|---|
| 1989 | IgG1 | Phosphonate ester | Leaf | ER | Tobacco |
| 1990 | IgM | NP hapten | Leaf | ER chloroplast | Tobacco |
| 1991 | VH domain | Substance P (neuropeptide) | Leaf | Intra- & extra-cellular | N. benthamiana |
| 1992 | scFv | Phytochrome | Leaf | Cytosol | Tobacco |
| 1993 | IgG1 Fab | Human creatine kinase | Leaf | Nucleolus | Tobacco |
| 1993 | scFv | Phytochrome | Leaf | Apoplast | Tobacco |
| 1993 | scFv | AMCV | Leaf | Cytosol | N. benthamiana |
| 1994 | IgG | Fungal cutinase | Root | Apoplast | Tobacco |
| 1994 | IgG1 | Streptococcus mutans adhesin | Leaf | Apoplast | Tobacco |
| 1995 | IgA/G | Streptococcus mutans adhesin | Leaf | Apoplast | Tobacco |
| 1995 | IgG | TMV | Leaf | Apoplast | Tobacco |
| 1996 | scFv | Cutinase | Leaf | ER | Tobacco |
| 1996 | IgM | RKN secretion | Leaf root | Apoplast | Tobacco |
| 1996 | scFv | BNYVV | Leaf | Apoplast | N. benthamiana |
| 1996 | scFv | Human creatine kinase | Leaf | Cytoplasm ER | Tobacco |
| 1996 | IgG1Fab | Human creatine kinase | Leaf | Apoplast | A. thaliana |
| 1997 | scFv | β-1,4-endoglucanase | Root | Chytosol | S. tuberosum |
| 1997 | scFv | Oxazolone | Leaf | ER | Tobacco |
| 1997 | scFv | Abscisic acid | Leaf | ER | Tobacco |
| 1997 | scFv | Abscisic acid | Seed | ER | Tobacco |
| 1997 | scFv-IT | CD-40 | Plant | Apoplast | Tobacco tissue culture |
| 1998 | scFv | Oxazolone | Tuber | ER | Potato |
| 1998 | Humanized IgG | HSV-2 | Plant | Secretory pathway | Soybean |
| 1998 | scFv | Dihydro-flavonol 4-reductase | Leaf | Sytosol | P. hybrida |
| 1999 | IgG | Human IgG | Plant | Apoplast | Alfalfa |
| 1999 | scFv | CEA | Leaf | Transient expression | Tobacco |
| 1999 | scFv | Topoviruses | Plant | ER, apoplast | N. benthamiana |
| 1999 | bi-scFv | TMV | Leaf | ER, apoplast | Tobacco cell susp. |
| 1999 | scFv | TMV | Plant | Cytosol | Tobacco |
| 1999 | scFv | CEA | Cell | Er, apoplast | Rice suspension cells |
| 1999 | scFv | 38C13 mouse B-cell lymphoma | Leaf | Apolost | N. benthamiana |
| 2000 | scFv | CEA | Plant | ER, apoloast | Rice, Wheat |
| 2000 | scFv | TMV | Leaf | Apoplast, membrin | Tobacco |
CEA, carcinoembryonic antigen; ER, endoplasmic reticulum; AMCV, artichoke mottle crinkle virus; TMV, tobacco mosaic virus; RKN, root knot nematode; BNYVV, beet necrotic yellow vein virus; HSV-2, herpes simplex virus-2; scFv-IT, scFv-bryodin-immunotoxin. N. benthamiana, tobacco (Nicotiana)-related species, A. thaliana, Arabidopsis, an experimental species. Table modified from Fischer & Emans.2
Antibodies derived from plants have a multitude of applications, including binding to pathogenic organisms, binding to serum or body fluid effector proteins (e.g. interleukins), binding to tumour antigens to deliver imaging or anti-tumour agents, or binding to a cellular receptor site to up- or down-regulate receptor function. However, plant glycosylation patterns differ from those in mammalian systems, and glycosylation is essential for antibody-mediated activation of complement or the initiation of cellular immune responses.11,22 Plantibodies may carry plant glycoproteins or may be non-glycosylated as a result of genetically deleting glycosylation sites, but are incapable of inducing the latter phenomena in either case.22 This does not appear to be a major limitation, however, since therapeutic applications of monoclonal antibodies are often mediated by binding and inactivation of proteins or receptor molecules and do not require complement or cell-mediated immunity. While glycosylation sequences are poorly immunogenic and hence unlikely to precipitate immunological adverse reactions,8 the presence of mammalian glycosylation sequences not required for therapeutic function may only serve to produce undesired complement- or cell-mediated side effects.
As of 2001, four antibodies expressed in plants had shown potential to be useful as therapeutics.3 A chimeric secretory IgG/IgA antibody effective against a surface antigen of Streptococcus mutans has been expressed in tobacco, and has been demonstrated to be effective against dental caries.24 Soybeans can express a humanized anti-herpes simplex virus (HSV), which has been effective in preventing the transmission of vaginal HSV-2 in animals.25 Rice and wheat expression systems can produce antibodies against carcinoembryonic antigen, which may be useful for in vivo tumor imaging.26 Finally, a plant viral vector has been used to produce a transiently expressed tumor-specific vaccine in tobacco for the treatment of lymphoma.27 Currently, seven plant-derived antibodies have reached the advanced stages of clinical product development.8 These include products directed at the treatment and/or diagnosis of cancer, dental caries, herpes simplex virus, and respiratory syncytial virus.
No ‘plantibodies’ have currently reached the commercialized stage, although at least one product has been tested clinically, and several have been examined in vitro and in animal systems and appear to be equivalent to mammalian-cell-derived analogues.28 Given the high levels of production, purification cost, apparent efficacy, and low immunogenicity of recombinant human antibodies derived from plants, plants appear to hold great potential for future production of monoclonal antibodies.
Vaccines
There has been considerable interest in developing low-cost, edible (i.e. oral) vaccines.29–32 Traditional edible vaccines, as for polio, use whole, attenuated organisms or semi-purified materials to induce both systemic (Ig-G-mediated) and local membrane (Ig-A-mediated) immunity. Plant vaccines can express entire selected proteins, but the use of DNA encoding only desired antigenic sequences from pathogenic viruses, bacteria and parasites has received considerable attention.33 Key immunogenic proteins or antigenic sequences can be synthesized in plant tissues and subsequently ingested as edible subunit vaccines.30,31,33 The mucosal immune system can induce protective immune responses against pathogens or toxins, and may also be useful to induce tolerance to ingested or inhaled antigens.30,31 The production of secretory Ig-A (sIg-A) and provocation of specific immune lymphocytes can occur in mucosal regions, and these regions take on special importance in the development of edible vaccines.
Aside from intrinsic low production cost, plant-based vaccines offer a number of unique advantages, including increased safety, stability, versatility, and efficacy.34 Plant produced vaccines can be grown locally where needed, avoiding storage and transportation costs. Relevant antigens are naturally stored in plant tissue, and oral vaccines can be effectively administered directly in the food product in which they are grown, eliminating purification costs.30,34 In many instances, it appears that refrigeration will not be needed to preserve vaccine efficacy, removing a major impediment to international vaccination efforts of the past.30,33 Plants engineered to express only a select antigenic portions of the relevant pathogen may reduce immunotoxicity and other adverse effects, and plant-derived vaccines are free of contamination with mammalian viruses. Finally, the development of multi-component vaccines is possible by insertion of multiple genetic elements or through cross-breeding of transgenic lines expressing antigens from various pathogenic organisms.
There are, however, some limitations associated with the use of transgenic plants for vaccine production.10 A major limitation of the expression of recombinant antigens in transgenic plants is obtaining a protein concentration adequate to confer total immunity, given varying protein expression among and within the various plant species. Tight control of expression yields will likely be necessary to reduce variability and assure consistent, effective immunization.10
During the last decade, nearly a dozen vaccine antigens have been expressed in plants (Table 3).2 Transgenic potatoes can produce antigens of enterotoxigenic E. coli heat labile enterotoxin B subunit, and is effective in immunizing against viruses and bacteria that cause diarrhoea. Still other ‘edible vaccines’ are under development for rabies, foot and mouth disease(veterinary), cholera, and autoimmune diabetes. Transgenic lupin and lettuce plants can express hepatitis B surface antigen. Efforts are underway to develop an ‘edible vaccine’ against the measles virus using the tobacco plant. A plant-based oral subunit vaccine for the respiratory syncytial virus (RSV) using either the apple or the tomato is under development.30
Recombinant vaccines expressed in plants
| Year . | Vaccine antigen . | Species . |
|---|---|---|
| 1992 | Hepatitis virus B surface antigen | Tobacco |
| 1995 | Malaria parasite antigen | Virus particle* |
| 1995 | Rabies virus glycoprotein | Tomato |
| 1995 | Escherichia coli heat-labile enterotoxin | Tobacco |
| Potato | ||
| 1996 | Human rhinovirus 14 (HRV-14) and human immunodeficiency virus type (HIV-1) epitopes | Virus particle* |
| 1996 | Norwalk virus capsid protein | Tobacco |
| Potato | ||
| 1997 | Diabetes-associated autoantigen | Tobacco |
| Potato | ||
| 1997 | Hepatitis B surface proteins | Potato |
| 1997 | Mink Enteritis Virus epitope | Virus particle* |
| 1997 | Rabies and HIV epitopes | Virus particle* |
| 1998 | Foot and mouth disease virus VP1 structural protein | Arabidopsis |
| 1998 | Escherichia coli heat-labile enterotoxin | Potato |
| 1998 | Escherichia coli heat-labile enterotoxin | Potato |
| 1998 | Rabies virus | Virus particle* |
| 1998 | Cholera toxin B subunit | Potato |
| 1998 | Human insulin-Cholera toxin B subunit fusion protein | Potato |
| 1999 | Foot and mouth disease virus VP1 structural protein | Alfalfa |
| 1999 | Hepatitis B virus surface antigen | Yellow lupin, lettuce |
| 1999 | Human cytomegalovirus glycoprotein B | Tobacco |
| 1999 | Dental caries (S. mutans) | Tobacco |
| 1999 | Diabetes-associated autoantigen | Tobacco |
| Carrot | ||
| 2002 | Respiratory syncytial virus | Tomato |
| Year . | Vaccine antigen . | Species . |
|---|---|---|
| 1992 | Hepatitis virus B surface antigen | Tobacco |
| 1995 | Malaria parasite antigen | Virus particle* |
| 1995 | Rabies virus glycoprotein | Tomato |
| 1995 | Escherichia coli heat-labile enterotoxin | Tobacco |
| Potato | ||
| 1996 | Human rhinovirus 14 (HRV-14) and human immunodeficiency virus type (HIV-1) epitopes | Virus particle* |
| 1996 | Norwalk virus capsid protein | Tobacco |
| Potato | ||
| 1997 | Diabetes-associated autoantigen | Tobacco |
| Potato | ||
| 1997 | Hepatitis B surface proteins | Potato |
| 1997 | Mink Enteritis Virus epitope | Virus particle* |
| 1997 | Rabies and HIV epitopes | Virus particle* |
| 1998 | Foot and mouth disease virus VP1 structural protein | Arabidopsis |
| 1998 | Escherichia coli heat-labile enterotoxin | Potato |
| 1998 | Escherichia coli heat-labile enterotoxin | Potato |
| 1998 | Rabies virus | Virus particle* |
| 1998 | Cholera toxin B subunit | Potato |
| 1998 | Human insulin-Cholera toxin B subunit fusion protein | Potato |
| 1999 | Foot and mouth disease virus VP1 structural protein | Alfalfa |
| 1999 | Hepatitis B virus surface antigen | Yellow lupin, lettuce |
| 1999 | Human cytomegalovirus glycoprotein B | Tobacco |
| 1999 | Dental caries (S. mutans) | Tobacco |
| 1999 | Diabetes-associated autoantigen | Tobacco |
| Carrot | ||
| 2002 | Respiratory syncytial virus | Tomato |
Recombinant vaccines expressed in plants
| Year . | Vaccine antigen . | Species . |
|---|---|---|
| 1992 | Hepatitis virus B surface antigen | Tobacco |
| 1995 | Malaria parasite antigen | Virus particle* |
| 1995 | Rabies virus glycoprotein | Tomato |
| 1995 | Escherichia coli heat-labile enterotoxin | Tobacco |
| Potato | ||
| 1996 | Human rhinovirus 14 (HRV-14) and human immunodeficiency virus type (HIV-1) epitopes | Virus particle* |
| 1996 | Norwalk virus capsid protein | Tobacco |
| Potato | ||
| 1997 | Diabetes-associated autoantigen | Tobacco |
| Potato | ||
| 1997 | Hepatitis B surface proteins | Potato |
| 1997 | Mink Enteritis Virus epitope | Virus particle* |
| 1997 | Rabies and HIV epitopes | Virus particle* |
| 1998 | Foot and mouth disease virus VP1 structural protein | Arabidopsis |
| 1998 | Escherichia coli heat-labile enterotoxin | Potato |
| 1998 | Escherichia coli heat-labile enterotoxin | Potato |
| 1998 | Rabies virus | Virus particle* |
| 1998 | Cholera toxin B subunit | Potato |
| 1998 | Human insulin-Cholera toxin B subunit fusion protein | Potato |
| 1999 | Foot and mouth disease virus VP1 structural protein | Alfalfa |
| 1999 | Hepatitis B virus surface antigen | Yellow lupin, lettuce |
| 1999 | Human cytomegalovirus glycoprotein B | Tobacco |
| 1999 | Dental caries (S. mutans) | Tobacco |
| 1999 | Diabetes-associated autoantigen | Tobacco |
| Carrot | ||
| 2002 | Respiratory syncytial virus | Tomato |
| Year . | Vaccine antigen . | Species . |
|---|---|---|
| 1992 | Hepatitis virus B surface antigen | Tobacco |
| 1995 | Malaria parasite antigen | Virus particle* |
| 1995 | Rabies virus glycoprotein | Tomato |
| 1995 | Escherichia coli heat-labile enterotoxin | Tobacco |
| Potato | ||
| 1996 | Human rhinovirus 14 (HRV-14) and human immunodeficiency virus type (HIV-1) epitopes | Virus particle* |
| 1996 | Norwalk virus capsid protein | Tobacco |
| Potato | ||
| 1997 | Diabetes-associated autoantigen | Tobacco |
| Potato | ||
| 1997 | Hepatitis B surface proteins | Potato |
| 1997 | Mink Enteritis Virus epitope | Virus particle* |
| 1997 | Rabies and HIV epitopes | Virus particle* |
| 1998 | Foot and mouth disease virus VP1 structural protein | Arabidopsis |
| 1998 | Escherichia coli heat-labile enterotoxin | Potato |
| 1998 | Escherichia coli heat-labile enterotoxin | Potato |
| 1998 | Rabies virus | Virus particle* |
| 1998 | Cholera toxin B subunit | Potato |
| 1998 | Human insulin-Cholera toxin B subunit fusion protein | Potato |
| 1999 | Foot and mouth disease virus VP1 structural protein | Alfalfa |
| 1999 | Hepatitis B virus surface antigen | Yellow lupin, lettuce |
| 1999 | Human cytomegalovirus glycoprotein B | Tobacco |
| 1999 | Dental caries (S. mutans) | Tobacco |
| 1999 | Diabetes-associated autoantigen | Tobacco |
| Carrot | ||
| 2002 | Respiratory syncytial virus | Tomato |
The plant species to be used for the production and delivery of an oral vaccine can be specifically selected to achieve desired goals. A large number of food plants (e.g. alfalfa, apple, asparagus, banana, barley, cabbage, canola, cantaloupe, carrots, cauliflower, cranberry, cucumber, eggplant, flax, grape, kiwi, lettuce, lupins, maize, melon, papaya, pea, peanut, pepper, plum, potato, raspberry, rice, service berry, soybean, squash, strawberry, sugar beet, sugarcane, sunflower, sweet potato, tomato, walnut, and wheat) have been transformed.29 Many of the high volume, high acreage plants such as corn, soybeans, rice, and wheat may offer advantages. Corn, since it is a major component in the diet of the domestic animal, is a good candidate for vaccine production. In humans, particularly infants, the plant of choice to produce the vaccine might be the banana. Bananas are a common component of many infant diets and can be consumed uncooked, thus eliminating the possibility of protein denaturation due to high temperatures. Unfortunately, it is relatively difficult to create transgenic bananas and the production time is longer than for certain other food crops. Cereals and other edible plants are advantageous for vaccine production over plant species such as tobacco because of the lower levels of toxic metabolites. It is evident that there are numerous opportunities to identify and develop low-cost plant derived vaccine materials, including edible plant-based vaccines.
Other therapeutic agents
A wide variety of other therapeutic agents have been derived from plants (Tables 4, 5), including hormones (somatotropin), enzymes, interleukins, interferons (IFN) and human serum albumin (HSA).2,23 Similar biotherapeutic agents have also been expressed from mammalian and bacterial cell systems.4 There is a worldwide demand for HSA, and plant production would offer the advantage of freedom from contamination with human pathogenic viruses. Modified rice plants are capable of producing human alpha-1-antitrypsin, a protein that may realize therapeutic potential in emphysema and hepatic diseases. Hirudin, originally isolated from leeches, is a blood anticoagulant that can now be expressed from oilseed rape, from tobacco and from mustard. Transgenic potato plants can encode for at least two subtypes of human INF, some of which may moderate certain cancers and diseases caused by viral agents.
Biopharmaceuticals derived from transgenic plants
| Potential application/indication . | Plant . | Protein . | Method . |
|---|---|---|---|
| Anticoagulants | |||
| Protein C pathway | Tobacco | Human protein C (serum protease) | AMT |
| Indirect thrombin inhibitors | Ethiopian mustard | Human hirudin variant 2 | AMT |
| Recombinant hormones/proteins | |||
| Neutropenia | Tobacco | Human granulocyte-macrophage colony-stimulating factor | AMT |
| Anaemia | Tobacco | Human erythropoietin | AMT |
| Antihyperanalgesic by opiate activity | Tobacco | Human erythropoietin | AMT |
| Wound repair/control of cell proliferation | Thale cress, oilseed | Human enkephalins | AMT |
| Hepatitis B and C treatment | Rice, turnip | Human interferon-α | AMT |
| Liver cirrhosis | Potato, tobacco | Human serum albumin | AMT |
| Blood substitute | Tobacco | Human haemoglobin | AMT |
| Collagen | Tobacco | Human homotrimeric collagen | AMT |
| Protein/peptide inhibitors | |||
| Cystic fibrosis, liver disease and haemorrhage | Rice | Human α-1-antitrypsin | PB |
| Trypsin inhibitor for transplantation surgery | Maize | Human aprotinin | PB |
| Hypertension | Tobacco/tomato | Antiotensin-1-converting enzyme | AMT |
| HIV therapies | Nicotiana bethamiana | α -Trichosanthin from TMV-U1 subgenomic coat protein | AMT |
| Recombinant enzymes | |||
| Gaucher's disease | Tobacco | Glucocerebrosidase | AMT |
| Potential application/indication . | Plant . | Protein . | Method . |
|---|---|---|---|
| Anticoagulants | |||
| Protein C pathway | Tobacco | Human protein C (serum protease) | AMT |
| Indirect thrombin inhibitors | Ethiopian mustard | Human hirudin variant 2 | AMT |
| Recombinant hormones/proteins | |||
| Neutropenia | Tobacco | Human granulocyte-macrophage colony-stimulating factor | AMT |
| Anaemia | Tobacco | Human erythropoietin | AMT |
| Antihyperanalgesic by opiate activity | Tobacco | Human erythropoietin | AMT |
| Wound repair/control of cell proliferation | Thale cress, oilseed | Human enkephalins | AMT |
| Hepatitis B and C treatment | Rice, turnip | Human interferon-α | AMT |
| Liver cirrhosis | Potato, tobacco | Human serum albumin | AMT |
| Blood substitute | Tobacco | Human haemoglobin | AMT |
| Collagen | Tobacco | Human homotrimeric collagen | AMT |
| Protein/peptide inhibitors | |||
| Cystic fibrosis, liver disease and haemorrhage | Rice | Human α-1-antitrypsin | PB |
| Trypsin inhibitor for transplantation surgery | Maize | Human aprotinin | PB |
| Hypertension | Tobacco/tomato | Antiotensin-1-converting enzyme | AMT |
| HIV therapies | Nicotiana bethamiana | α -Trichosanthin from TMV-U1 subgenomic coat protein | AMT |
| Recombinant enzymes | |||
| Gaucher's disease | Tobacco | Glucocerebrosidase | AMT |
Biopharmaceuticals derived from transgenic plants
| Potential application/indication . | Plant . | Protein . | Method . |
|---|---|---|---|
| Anticoagulants | |||
| Protein C pathway | Tobacco | Human protein C (serum protease) | AMT |
| Indirect thrombin inhibitors | Ethiopian mustard | Human hirudin variant 2 | AMT |
| Recombinant hormones/proteins | |||
| Neutropenia | Tobacco | Human granulocyte-macrophage colony-stimulating factor | AMT |
| Anaemia | Tobacco | Human erythropoietin | AMT |
| Antihyperanalgesic by opiate activity | Tobacco | Human erythropoietin | AMT |
| Wound repair/control of cell proliferation | Thale cress, oilseed | Human enkephalins | AMT |
| Hepatitis B and C treatment | Rice, turnip | Human interferon-α | AMT |
| Liver cirrhosis | Potato, tobacco | Human serum albumin | AMT |
| Blood substitute | Tobacco | Human haemoglobin | AMT |
| Collagen | Tobacco | Human homotrimeric collagen | AMT |
| Protein/peptide inhibitors | |||
| Cystic fibrosis, liver disease and haemorrhage | Rice | Human α-1-antitrypsin | PB |
| Trypsin inhibitor for transplantation surgery | Maize | Human aprotinin | PB |
| Hypertension | Tobacco/tomato | Antiotensin-1-converting enzyme | AMT |
| HIV therapies | Nicotiana bethamiana | α -Trichosanthin from TMV-U1 subgenomic coat protein | AMT |
| Recombinant enzymes | |||
| Gaucher's disease | Tobacco | Glucocerebrosidase | AMT |
| Potential application/indication . | Plant . | Protein . | Method . |
|---|---|---|---|
| Anticoagulants | |||
| Protein C pathway | Tobacco | Human protein C (serum protease) | AMT |
| Indirect thrombin inhibitors | Ethiopian mustard | Human hirudin variant 2 | AMT |
| Recombinant hormones/proteins | |||
| Neutropenia | Tobacco | Human granulocyte-macrophage colony-stimulating factor | AMT |
| Anaemia | Tobacco | Human erythropoietin | AMT |
| Antihyperanalgesic by opiate activity | Tobacco | Human erythropoietin | AMT |
| Wound repair/control of cell proliferation | Thale cress, oilseed | Human enkephalins | AMT |
| Hepatitis B and C treatment | Rice, turnip | Human interferon-α | AMT |
| Liver cirrhosis | Potato, tobacco | Human serum albumin | AMT |
| Blood substitute | Tobacco | Human haemoglobin | AMT |
| Collagen | Tobacco | Human homotrimeric collagen | AMT |
| Protein/peptide inhibitors | |||
| Cystic fibrosis, liver disease and haemorrhage | Rice | Human α-1-antitrypsin | PB |
| Trypsin inhibitor for transplantation surgery | Maize | Human aprotinin | PB |
| Hypertension | Tobacco/tomato | Antiotensin-1-converting enzyme | AMT |
| HIV therapies | Nicotiana bethamiana | α -Trichosanthin from TMV-U1 subgenomic coat protein | AMT |
| Recombinant enzymes | |||
| Gaucher's disease | Tobacco | Glucocerebrosidase | AMT |
Selected pharmaceutical proteins expressed in transgenic plants
| Year . | Protein . | Species . |
|---|---|---|
| 1986 | Human growth hormone | Tobacco |
| Sunflower | ||
| 1990 | Human serum albumin | Tobacco |
| Potato | ||
| 1993 | Human epidermal growth factor | Tobacco |
| 1994 | Trout growth factor | Tobacco |
| 1994 | Human α-interferon | Rice |
| 1995 | Hirudin | Tobacco |
| Suspension cells | ||
| 1995 | Erythropoietin | Tobacco |
| Suspension cells | ||
| 1996 | Glucocerebrosidase, human protein C serum protease | Tobacco |
| 1997 | Human α and β haemoglobin | Tobacco |
| 1997 | Human muscarinic cholinergic receptors | Tobacco |
| 1997 | Murine granulocyte- macrophage colony stimulating factor | Tobacco |
| 1998 | Interleukin-2 and interleukin-4 | Tobacco |
| Suspension cells | ||
| 1999 | Human placental alkaline phosphatase | Tobacco |
| Rhizosecretion | ||
| 1999 | Human α-l-antitrypsin | Rice |
| Suspension cells | ||
| 2000 | Human growth hormone (somatotropin) | Tobacco seeds |
| 2000 | Human growth hormone (somatotropin) | Tobacco chloroplasts |
| Year . | Protein . | Species . |
|---|---|---|
| 1986 | Human growth hormone | Tobacco |
| Sunflower | ||
| 1990 | Human serum albumin | Tobacco |
| Potato | ||
| 1993 | Human epidermal growth factor | Tobacco |
| 1994 | Trout growth factor | Tobacco |
| 1994 | Human α-interferon | Rice |
| 1995 | Hirudin | Tobacco |
| Suspension cells | ||
| 1995 | Erythropoietin | Tobacco |
| Suspension cells | ||
| 1996 | Glucocerebrosidase, human protein C serum protease | Tobacco |
| 1997 | Human α and β haemoglobin | Tobacco |
| 1997 | Human muscarinic cholinergic receptors | Tobacco |
| 1997 | Murine granulocyte- macrophage colony stimulating factor | Tobacco |
| 1998 | Interleukin-2 and interleukin-4 | Tobacco |
| Suspension cells | ||
| 1999 | Human placental alkaline phosphatase | Tobacco |
| Rhizosecretion | ||
| 1999 | Human α-l-antitrypsin | Rice |
| Suspension cells | ||
| 2000 | Human growth hormone (somatotropin) | Tobacco seeds |
| 2000 | Human growth hormone (somatotropin) | Tobacco chloroplasts |
Modified from Fischer & Emans.2
Selected pharmaceutical proteins expressed in transgenic plants
| Year . | Protein . | Species . |
|---|---|---|
| 1986 | Human growth hormone | Tobacco |
| Sunflower | ||
| 1990 | Human serum albumin | Tobacco |
| Potato | ||
| 1993 | Human epidermal growth factor | Tobacco |
| 1994 | Trout growth factor | Tobacco |
| 1994 | Human α-interferon | Rice |
| 1995 | Hirudin | Tobacco |
| Suspension cells | ||
| 1995 | Erythropoietin | Tobacco |
| Suspension cells | ||
| 1996 | Glucocerebrosidase, human protein C serum protease | Tobacco |
| 1997 | Human α and β haemoglobin | Tobacco |
| 1997 | Human muscarinic cholinergic receptors | Tobacco |
| 1997 | Murine granulocyte- macrophage colony stimulating factor | Tobacco |
| 1998 | Interleukin-2 and interleukin-4 | Tobacco |
| Suspension cells | ||
| 1999 | Human placental alkaline phosphatase | Tobacco |
| Rhizosecretion | ||
| 1999 | Human α-l-antitrypsin | Rice |
| Suspension cells | ||
| 2000 | Human growth hormone (somatotropin) | Tobacco seeds |
| 2000 | Human growth hormone (somatotropin) | Tobacco chloroplasts |
| Year . | Protein . | Species . |
|---|---|---|
| 1986 | Human growth hormone | Tobacco |
| Sunflower | ||
| 1990 | Human serum albumin | Tobacco |
| Potato | ||
| 1993 | Human epidermal growth factor | Tobacco |
| 1994 | Trout growth factor | Tobacco |
| 1994 | Human α-interferon | Rice |
| 1995 | Hirudin | Tobacco |
| Suspension cells | ||
| 1995 | Erythropoietin | Tobacco |
| Suspension cells | ||
| 1996 | Glucocerebrosidase, human protein C serum protease | Tobacco |
| 1997 | Human α and β haemoglobin | Tobacco |
| 1997 | Human muscarinic cholinergic receptors | Tobacco |
| 1997 | Murine granulocyte- macrophage colony stimulating factor | Tobacco |
| 1998 | Interleukin-2 and interleukin-4 | Tobacco |
| Suspension cells | ||
| 1999 | Human placental alkaline phosphatase | Tobacco |
| Rhizosecretion | ||
| 1999 | Human α-l-antitrypsin | Rice |
| Suspension cells | ||
| 2000 | Human growth hormone (somatotropin) | Tobacco seeds |
| 2000 | Human growth hormone (somatotropin) | Tobacco chloroplasts |
Modified from Fischer & Emans.2
Erythropoietin (EPO) an also be expressed in transgenic tobacco plants. Erythropoietin, a glycoprotein used to treat anaemias, was commercialized from mammalian systems nearly 20 years ago. Blood substitutes such as human haemoglobin have long been pursued, and human haemoglobin derived from transgenic tobacco is being tested to ensure the molecule's function and oxygen-carrying capacity.35
In general, the levels of pharmaceutical proteins produced by transgenic plants have been low, often <1% of total soluble protein. While this is quite sufficient to allow for economical production of highly active pharmaceutical molecules, improved technologies for high level expression of protein will be probably needed to allow practical production of high volume human replacement proteins such as HSA,3 haemoglobin or blood coagulation factors.
Future directions
The use of plants as factories for the production of novel vaccines, antibodies and other therapeutic proteins will undoubtedly continue to develop. Molecular farming may become the premier expression system for a wide variety of new biopharmaceuticals and ‘plantibodies’. Important economic advantages will likely be realized as the technology continues to evolve and improve. Efforts will need to focus on increasing yields, on scale-up of production, on distribution and handling of transgenic plant material, and on the development and validation of production techniques which effectively isolate pharmaceutical production from human and animal food.
Plant-derived biopharmaceuticals will need to meet the same safety and efficacy standards as those products obtained from non-plant sources. There will be a need for continued vigilance to safeguard the environment, ensuring that errant substances do not affect non-target organisms. Gene containment methodologies will continue to develop, and there must be safeguards against the over-expression of potentially harmful proteins in transgenic pollen.
Undoubtedly, there will be a continuing debate about the use of transgenic food plants, as opposed to non-food plants, for producing new pharmaceuticals.
The advantages of recombinant plant DNA technology for the production of antibodies, vaccines, other pharmaceuticals, and even high-volume plasma proteins are becoming increasingly apparent. As the technology involves, it appears highly likely that plant-derived pharmaceuticals will play a significant role in the future of clinical therapeutics.
Dr Goldstein is a full-time employee of The Monsanto Company, St. Louis, Missouri. Monsanto previously engaged in research directed at the commercial production of plant-made-pharmaceuticals, but discontinued all efforts in this area in October of 2003. Dr Thomas has served from time to time as a consultant to Monsanto in the areas of plant biotechnology and food safety assessment, and did receive remuneration for his efforts in preparing this manuscript.
References
Daniell H, Streatfield SJ, Wycoff K. Medical molecular farming: production of antibodies, biopharmaceuticals and edible vaccines in plants.
Thomas JA. Biotechnology: Safety evaluation of biotherapeutics and agribiotechnology products. In: Thomas JA, Fuchs RL, eds.
PhRMA. 2002 Survey—New medicines in development—Biotechnology. Washington DC, Pharmaceutical Manufacturers Association of America, 2002. [www.pharma.org/newmedicines/resources/2002-10-21.93.pdf]
HighTech Business Decisions Report.
Ma JKC, Drake PMW, Christou P. The production of recombinant pharmaceutical proteins in plants.
Humphreys DP, Glover DJ. Therapeutic antibody production technologies: molecules, applications, expression, and purification.
Chargelegue D, Obregon P, Drake PMW. Transgenic plants for vaccine production: Expectations and limitations.
Russell DA. Feasibility of antibody production in plants for human therapeutic use.
BIO (Biotechnology Industry Organization).
Panchagnula R, Thomas NS. Biopharmaceutics and pharmacokinetics in drug research.
Thomas JA. Recent developments and perspectives of biotechnology-derived products.
Fischer R, Hoffmann K, Schillberg A, Emans N. Antibody production by molecular farming in plants.
Fraley RT, Rogers SG, Horsch RB, et al. Expression of bacterial genes in plant cells.
Horsch R, Fry JE, Hoffman N, Eicholtz D, Rogers S, Fraley R. A simple and general method for transferring genes into plants.
Barta A, Sommergruber K, Thompson D, Hartmuth K, Matzke M, Matzke A. The expression of a nopaline synthase- human growth hormone chimeric gene in transformed tobacco and sunflower callus tissue.
DeZoeten GA, Penswick JR, Horisberger MA, Ahl P, Schultze M, Hohn T. The expression, localization, and effect of a human interferon in plants.
Hiatt A, Cafferkey R, Bowdish K. The production of antibodies in transgenic plants.
Sijmons OC, Dekker BMM, Schrammeijer B, Vorwoerd RC, van den Elzen PJM, Hoekema A. Production of correctly processed human serum albumin in transgenic plants.
Ma JK, Hikmat BY, Wycoff K, Vine ND, Chargelegue D, Yu L, Hein MB, Lehner T. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans.
Zeitlin L, Olmsted SS, Moench TR, Co MS, Martinell BJ, Paradkar VM, Russell DR, Queen C, Cone RA, Whaley KJ. A humanized monoclonal antibody produced in transgenic plants for immunoprotection of the vagina against genital herpes.
Stoger E, Vaquero C, Torres E, Sack M, Nicholson L, Drossard J, Williams S, Keen D, Perrin Y, Christou P, Fischer R. Cereal crops as viable production and storage systems for pharmaceutical scFv antibodies.
McCormick AA, Kumagai MH, Hanley K, Turpen TH, Hakim I, Grill LK, Tuse D, Levy S, Levy R. Rapid production of specific vaccines for lymphoma by expression of the tumor-derived single-chain Fv epitopes in tobacco plants.
Richter L, Kipp PB. Transgenic plants as edible vaccines.
Korban SS, Krasnyanski SF, Buetow DE. Foods as production and delivery vehicles for human vaccines.
Tacket CO, Mason HS. A review of oral vaccination with transgenic vegetables.
Giddings G, Allison G, Brooks D, Carter A. Transgenic plants as factories for biopharmaceuticals.
Mahon BR, Moore A, Johnson PA, Mills KHG. Approaches to new vaccines.
Streatfield SJ, Jilka JM, Hood EE, Turner DD, Baily MR, Mayor JM, Woodard SL, Beifuss KK, Horn ME, Delaney DE, Tizard IR, Howard JA. Plant-based vaccines: unique advantages.
Author notes
From the 1Monsanto Company, St Louis, and 2The University of Texas Health Science Center, San Antonio, USA