-
PDF
- Split View
-
Views
-
Cite
Cite
I. Marie, G. Sauvetre, H. Levesque, Small intestinal involvement revealing sarcoidosis, QJM: An International Journal of Medicine, Volume 103, Issue 1, January 2010, Pages 60–62, https://doi.org/10.1093/qjmed/hcp135
Close - Share Icon Share
Sir,
Sarcoidosis is an immune-mediated condition affecting multiple organs, especially the lungs, lymph nodes, skin and eyes.1,2 Gastrointestinal involvement is considered to be a rare complication of sarcoidosis, occurring in <10% of the patients during the course of the disease.3,4 We recently observed the interesting case of a patient who developed duodenal involvement as the first manifestation of sarcoidosis.
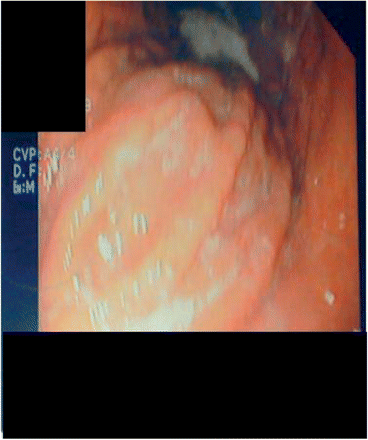
A 36-year-old woman, with unremarkable previous medical history, presented with a 4-month history of asthenia and a 10-kg weight loss; she also complained of abdominal pain, diarrhea and arthralgia. At admission, physical examination demonstrated abdominal pain to palpation, involving the epigastric area; general physical examination was otherwise normal. Laboratory findings disclosed the following: erythrocyte sedimentation rate 66 mm/h; C-reactive protein 25 mg/l; hemoglobin 12 g/dl; white blood cell count 9.1 × 109/l; and platelet count 450 × 109/l. Other routine biochemical tests, including renal and liver tests, vitamin B12 and folic acid blood levels, as well as blood and urinary protein immunoelectrophoresis, were normal. Stool cultures (Yersinia, Salmonella, Camplylobacter and Shigella) yielded negative results. Because of abdominal clinical manifestations, gastroscopy was performed, which showed granulomatous duodenal involvement (Figure 1). Histological examination of duodenal lesion specimens showed numerous non-caseating epithelioid granulomas; there was no evidence of Helicobacter pylori. Special stains and cultures of the duodenum biopsy specimens were negative for Mycobacterium species and fungi; polymerase chain reaction amplification of Mycobacterium tuberculosis complex and bacterial 16S ribosomal RNA further proved negative. Tuberculin skin test was negative; further microbiological studies (Ziehl–Neelsen stain and cultures) in sputum and urine products were also negative for acid-fast bacilli. Thoracic and abdominal computed tomography-scan showed bilateral hilar lymphadenopathy. Pulmonary function tests revealed a mild decrease of diffusing capacity for carbon monoxide (68%) without restrictive pattern. Bronchoscopy was systematically performed; histological examination also showed numerous non-caseating epithelioid granulomas. Bacterial (Treponema pallidum) and viral (cytomegalovirus, Epstein–Barr virus, Herpes simplex virus, hepatitis B and C, human immunodeficiency virus) were negative. Autoantibody screening also yielded negative results for rheumatoid factors, anti-nuclear and anti-neutrophil cytoplasmic antibodies, anti-Saccharomyces cerevisae antibodies, anti-endomysium and anti-transglutaminase antibodies; serum angiotensin-converting enzyme level was elevated: 97 IU/l (n < 68). Other investigations, including accessory salivary gland histological examination, colonoscopy and small bowel barium meal were normal. A definite diagnosis of small intestinal involvement related to sarcoidosis was made. Because of the systemic manifestations related to sarcoidosis, steroid therapy, at an initial dose of 30 mg daily was initiated. The patient's condition improved rapidly with regression of clinical symptoms and weight gain.
Gastrointestinal involvement is rare in sarcoidosis. Previous autopsy series have, in fact, found no gastrointestinal impairment in patients with sarcoidosis,5 while other authors have observed subclinical gastrointestinal sarcoidosis in 5–10% of the patients.2,3 The stomach, particularly the antrum, is the most frequent extra-hepatic organ to be involved; on the other hand, the small intestine is the least common.2,3 Intestinal involvement usually occurs in patients with chronic multisystem sarcoidosis, although it may be the first manifestation of the disease;2,3 in our patient, small bowel impairment revealed sarcoidosis. Clinical manifestations of sarcoidosis-related intestinal impairment are non-specific, including epigastric and peri-umbilical pain, diarrhea, weight loss, malabsorption, protein-losing enteropathy or duodenal obstruction.2,3,6,7 Nevertheless, the definite diagnosis of sarcoidosis-related small intestinal impairment can only be established after exclusion of other conditions.8 In our patient, a diagnosis of sarcoidosis-related small intestinal involvement could be made as: (i) the extensive search for other conditions was unsuccessful, including infections (tuberculosis, Whipple's disease and syphilis), Crohn's disease, celiac disease, foreign body reaction, autoimmune diseases, as well as drug-related lesions and malignancy (lymphoma, carcinoma); (ii) there was a combination of the histological granulomatous duodenal involvement and the pulmonary lesions; and (iii) the resolution of clinical manifestations took place after initiation of steroid therapy. Our findings underline that when unexplained intestinal manifestations are noted, careful clinical examination should systematically be performed in order to detect underlying sarcoidosis. Moreover, our data also indicate that patients with sarcoidosis who exhibit persistent diarrhea and abdominal pain should be investigated for intestinal sarcoidosis. Therapy of sarcoidosis-related small intestinal involvement remains controversial. Previous authors have suggested that steroids should be considered in the group of the patients who exhibit: (i) symptomatic intestinal lesions and (ii) intestinal involvement associated with multivisceral and severe sarcoidosis. The recommended starting dose of steroid therapy is 20–40 mg daily, with gradual dose decrease subsequently.9,10 Steroid therapy has been reported to improve intestinal gastrointestinal symptoms in 66% of the patients. 9,10
Finally, our case report reinforces the possibility of an unusual presentation of sarcoidosis. Indeed, physicians should be aware that small bowel involvement may be related to sarcoidosis, resulting in earlier diagnosis and management. In such patients exhibiting unusual intestinal manifestations, diagnosis of sarcoidosis relies on compatible clinical signs, evidence of non-caseating granulomas and exclusion of underlying conditions, especially infections and other granulomatous diseases. Moreover, because small intestinal involvement may precede other signs of sarcoidosis, our findings indicate that when unexplained intestinal involvement is noted, an evaluation for sarcoidosis should be made.