-
PDF
- Split View
-
Views
-
Cite
Cite
David E.J. Jones, Katy Sutcliffe, Jessie Pairman, Katharine Wilton, Julia L. Newton, An integrated care pathway improves quality of life in Primary Biliary Cirrhosis, QJM: An International Journal of Medicine, Volume 101, Issue 7, July 2008, Pages 535–543, https://doi.org/10.1093/qjmed/hcn043
Close - Share Icon Share
Abstract
Background: Clinical management of the chronic autoimmune liver disease, Primary Biliary Cirrhosis (PBC) involves addressing the underlying liver disease and a range of symptoms independent of liver disease severity. We have formally explored how these two perspectives of chronic disease management can be combined into a clinic consultation and impact upon quality of life (QOL) in PBC.
Aims: To develop and implement the first Integrated Care Pathway (ICP) for the management of liver disease progression and symptom management in PBC.
Methods: Process mapping of current practice by a multidisciplinary group developed a flowchart of care from which the clinical record evolved. Symptom assessment is incorporated into the PBC ICP (QOL; PBC-40, autonomic symptoms; Orthostatic Grading Scale, daytime sleepiness; Epworth Sleepiness Scale). All patients were considered who attended clinic between July 2005 and June 2006. Symptom assessment was repeated after 1 year in those participating in the initial clinic cohort.
Results: The PBC ICP was successfully introduced into our clinical environment with high levels of patient satisfaction. A total of 225 PBC patients attended over 12 months. Initial QOL assessments were in 195 (87%). Five patients died (3%). Repeat assessment 1 year later occurred in 149 subjects (149/190; 78%). All symptom domains improved after ICP implementation with significant improvements in those with moderate and severe symptoms in all PBC-40 symptom domains (P < 0.02). In those with severe fatigue (n = 38) symptom improvement was even more dramatic (P = 0.002).
Conclusion: ICP implementation delivers evidence-based care, leads to improvements in QOL coupled with high levels of patient satisfaction.
Introduction
Historically, clinical management of the chronic autoimmune liver disease Primary Biliary Cirrhosis (PBC) has focussed upon slowing down progression of the liver disease, identifying those at risk of deterioration and management of the complications of advanced liver disease.1 Recent studies suggest that, in addition, PBC patients experience a range of symptoms that are independent of the severity of the underlying liver disease and represent an important clinical entity in their own right.2–6 The means by which these two perspectives of PBC disease management can be combined into a clinic consultation and whether this type of standardized approach improves the quality of life (QOL) of patients with PBC has not previously been explored.
In the Newcastle PBC Service, we have developed the first PBC Integrated Care Pathway (ICP) for the management of PBC which incorporates the currently available evidence-based management. This document was developed by a multi-disciplinary group that included representation from the patient support group and was piloted in our Specialist PBC Clinic. The purpose of an ICP is to determine locally agreed, multidisciplinary practice based on guidelines and evidence where available, for a specific patient/user group. It forms all or part of the clinical record, documents the care given and facilitates the evaluation of outcomes for continuous quality improvement.7,8 An ICP has the potential to be an excellent tool for use in patients attending a PBC service either as part of a specialist clinic or to guide general physicians who rarely see patients with this relatively uncommon condition. Our experiences of the development of this documentation and its implementation are described, and the positive impact of its use upon the symptomatology experienced by our PBC clinic cohort is explored.
Methods
Development of the Newcastle Primary Biliary Cirrhosis ICP
The driver within the Newcastle PBC service is the provision of high quality, evidence-based health care, delivered in a healthy and safe environment by well-trained and highly motivated staff. The service was keen to ensure standardization of the delivery and quality of care both internally and externally. In addition the motivators for development of such a disease-specific ICP were recognized as enhancing the opportunity for dissemination, education and training.
A sub-group was formed involving medical staff (Consultant), nursing sister, database manager, our Hospital Trusts ICP co-ordinator and a member of the local patient support group, LIVErNORTH. This subgroup allocated process mapping of current practice to relevant members of the multidisciplinary group, who then gave feed back to the sub-group (Figure 1). In the first instance the working group examined current practice including: evidence base/best practice, Guidelines, protocols, investigations, patient information written/verbal and outcomes/goals.
The group then set about: This process allowed development of a flow-chart of care and then, based upon this, the clinical record evolved. This document was then taken through a process of staff education (to ensure continued positive support by all staff, allow ownership of the record by staff, and ensure appropriate completion of the record), implementation and reassessment on a 3-monthly basis. The clinical record was revised and refined within the entire staff group and sub-group on a 3-monthly basis for 9 months. At the end of this pilot stage, the ultimate clinical record was finalized. The PBC ICP first came into use in our PBC clinic in July 2005.
Process mapping the route of new patient's first and subsequent follow-up visits to the PBC Service.
Specific conditions/diagnosis
Medications prescribed
Specific Investigations
Outcomes/goals
This entire project is registered with our organization as on-going service development (as can be seen in the ICP) patients consent to the use of the data collected during their clinic appointments for service development. Newcastle and North Tyneside Local Research Ethics Committee and Caldicott Guardian have reviewed the use of this data and agree that its use constitutes service development and therefore does not require ethical permission for publication.
Algorithm development
During the development of the ICP it became clear that numerous management areas in patients with PBC were consistent. As a result, a series of management algorithms were developed and workshoped. As a result the ICP was based upon the following series of clinical management processes which involved both the management of the underlying liver disease and also the symptoms commonly associated with the liver disease. These were:
Monitoring of liver disease stage.
Identification of the complications of advanced liver disease, specifically varices and hepatocellular carcinoma.
Detection and management of osteoporosis.9
Detection and management of overlap with autoimmune liver disease.10
Detection and management of hypercholesterolaemia.
Detection and management of itch.10
Use and evaluation of the PBC ICP
In order to explore the impact of the PBC ICP upon the patient experience, all patients attending our specialist PBC clinic between July 2005 and June 2006 were invited to perform a series of assessments of their QOL (Initial assessment). In order to be eligible for inclusion in the study cohort, patients had to have definite (all three of anti-mitochondrial antibody or PBC-specific anti-nuclear antibody at a titre of ⩾1:40 by immuno-fluorescence, cholestatic liver function tests and compatible liver histology) or probable (two out of the three above criteria) disease as previously defined.12,13 One year later, all PBC patients in the clinic cohort were sent by post the same symptom assessment tools to complete and return in a prepaid envelope (Repeat assessment). Patients were unaware of the purposes of these questionnaires, and to what the results will be compared.
The study was approved by the local research ethics committee and consent for the use of data implied by return of questionnaires. All data were coded and returned anonymously.
Symptom assessment tools
All the symptom assessment tools used in our clinical practise have been validated for self completion and for use in PBC.
PBC-40
Health-related QOL and the symptoms which contribute to its impairment in PBC were assessed in the PBC patient cohort using the PBC-40, a fully validated PBC-specific, multi-domain, QOL measure.14 The PBC-40 contains 40 questions in five domains [Fatigue, Itch, Cognitive, Social and Emotional and Other Symptoms (a domain relating to a number of PBC-related symptoms which did not map to the other domains including appetite, symptoms related to consumption of alcohol, right sided discomfort, dry eyes and dry mouth, aches in the long bones)]. Previous studies performed during the validation of the PBC-40 demonstrated strong correlations between individual Fatigue domain and Other Symptom, Cognitive, Social and Emotional domain scores (which, in turn all correlated with each other), but no correlation with Itch scores.15 Participants rate items on a 5-point scale (1 = ‘never’, 2 = ‘rarely’, 3 = ‘sometimes’, 4 = ‘most of the time’, 5 = ‘always’). Previous work has developed clinically meaningful bands for symptom severity. Changes in symptoms were considered in those with moderate and severe symptoms, defined as previously.16
Orthostatic Grading Scale
Studies suggest a role for autonomic dysfunction and fatigue in both non-hepatic diseases and PBC. Subjects therefore completed the Orthostatic Grading Scale (OGS), a fully validated self-report assessment tool for the symptoms of orthostatic intolerance due to orthostatic hypotension (e.g. severity, frequency and interference with daily activities) which consists of five items, each graded on a scale of 0–4. Adding the scores for the individual items creates a total score. Studies have shown that scores from the OGS correlate with conventional tests of the integrity of the autonomic nervous system. A score >9 is considered to be consistent with a formal diagnosis of orthostatic hypotension. A score of ⩾ 4 and <9 was regarded as being indicative of moderate orthostatic hypotension.17
Epworth Sleepiness Scale
In view of the recently identified associations between excessive daytime sleep and fatigue in PBC in selected cohorts of clinic patients18 all subjects completed the Epworth Sleepiness Scale (ESS, possible score range 0–24). This fully validated tool assesses day-time hyper-somnolence, a score of 10 or more being indicative of significant daytime hyper-somnolence. A score of ⩾5 and <10 was regarded as being indicative of moderate daytime hyper-somnolence.19
Data analysis
All data were normally distributed and are presented as mean and standard deviation. Data were analysed using Graphpad software (Prism). Comparisons between groups were by student's paired and unpaired t-tests as appropriate. Correlations between variables were by Spearman Rank test.
Results
Implementation of the PBC ICP
The Newcastle PBC ICP was successfully introduced into our clinical environment with high levels of patient satisfaction and no complaints from patients or carers. This document is available from the authors or to be downloaded from the National Library for Health, UK (www.nlh.org.uk).
The impact of implementation of the PBC ICP upon patient QOL
A total of 225 PBC patients attended our Clinic over the 12-month period. The demographic details of this patient cohort are shown in Table 1. Initial QOL assessments were available and complete in 195 patients during that period (87%). During follow-up five patients in the clinic cohort died (3%), there were no significant differences in symptom scores at initial assessment between those who died and those who did not (Table 2). In this group, two patients died from liver related deaths, two patients died from non-liver related cancer and one from sudden cardiac death. Repeat assessment was available (i.e. paired data) 1 year after initial assessment in 149 of the PBC subjects who were alive (149/190; 78%). There were no significant differences in QOL in those who completed and returned the questionnaires and those who did not (Table 2). In addition, there were no relationships between years of diagnosis and symptom scores (all P > 0.1; r2 < 0.01). Furthermore, biochemical markers of liver disease severity did not change significantly during follow-up (Bilirubin at baseline 12 ± 15 vs. follow-up 13 ± 15; P = 1.0, Albumin 41 ± 4.1 vs. 40 ± 5; P = 0.2, Alanine transaminase 42 ± 35 vs. 50 ± 78; P = 0.4, Alkaline phosphatase 168 ± 190 vs. 180 ± 149; P = 0.5) and as in previous studies there was no relationship between these markers and QOL measures.
Demographic details of PBC Clinic Cohort included in the follow-up assessment of quality of life. All values are shown as mean ± SD unless otherwise stated
| Characteristics . | Value . |
|---|---|
| . | |
| n | 225 |
| Females n (%) | 218 (97%) |
| Age (years) | 63 ± 12 |
| Length of time since diagnosis (years) | 10 ± 12 |
| Blood parameters | |
| Bilirubin | 12 ± 15 |
| ALT | 42 ± 35 |
| ALP | 168 ± 190 |
| Albumin | 41 ± 4.1 |
| Platelets | 250 ± 94 |
| TSH | 2.2 ± 14 |
| IGG | 13 ± 11 |
| Histology | |
| No biopsy (%)/Precirrhotic/cirrhotic | 119 (53%)/ 64 (28%)/42 (19%) |
| Medication | |
| UDCA | 124 (55%) |
| Mean daily dose | 623 mg |
| Potentially culprit medication | 77 (34%) |
| Symptoms described by patient | |
| Loss of consiousness | 16 (7%) |
| Fatigue | 128 (57%) |
| Itch | 71 (31%) |
| Dry eyes | 83 (37%) |
| Dry mouth | 99 (44%) |
| Postural dizziness | 79 (35%) |
| Unexplained Falls | 14 (6%) |
| Characteristics . | Value . |
|---|---|
| . | |
| n | 225 |
| Females n (%) | 218 (97%) |
| Age (years) | 63 ± 12 |
| Length of time since diagnosis (years) | 10 ± 12 |
| Blood parameters | |
| Bilirubin | 12 ± 15 |
| ALT | 42 ± 35 |
| ALP | 168 ± 190 |
| Albumin | 41 ± 4.1 |
| Platelets | 250 ± 94 |
| TSH | 2.2 ± 14 |
| IGG | 13 ± 11 |
| Histology | |
| No biopsy (%)/Precirrhotic/cirrhotic | 119 (53%)/ 64 (28%)/42 (19%) |
| Medication | |
| UDCA | 124 (55%) |
| Mean daily dose | 623 mg |
| Potentially culprit medication | 77 (34%) |
| Symptoms described by patient | |
| Loss of consiousness | 16 (7%) |
| Fatigue | 128 (57%) |
| Itch | 71 (31%) |
| Dry eyes | 83 (37%) |
| Dry mouth | 99 (44%) |
| Postural dizziness | 79 (35%) |
| Unexplained Falls | 14 (6%) |
Demographic details of PBC Clinic Cohort included in the follow-up assessment of quality of life. All values are shown as mean ± SD unless otherwise stated
| Characteristics . | Value . |
|---|---|
| . | |
| n | 225 |
| Females n (%) | 218 (97%) |
| Age (years) | 63 ± 12 |
| Length of time since diagnosis (years) | 10 ± 12 |
| Blood parameters | |
| Bilirubin | 12 ± 15 |
| ALT | 42 ± 35 |
| ALP | 168 ± 190 |
| Albumin | 41 ± 4.1 |
| Platelets | 250 ± 94 |
| TSH | 2.2 ± 14 |
| IGG | 13 ± 11 |
| Histology | |
| No biopsy (%)/Precirrhotic/cirrhotic | 119 (53%)/ 64 (28%)/42 (19%) |
| Medication | |
| UDCA | 124 (55%) |
| Mean daily dose | 623 mg |
| Potentially culprit medication | 77 (34%) |
| Symptoms described by patient | |
| Loss of consiousness | 16 (7%) |
| Fatigue | 128 (57%) |
| Itch | 71 (31%) |
| Dry eyes | 83 (37%) |
| Dry mouth | 99 (44%) |
| Postural dizziness | 79 (35%) |
| Unexplained Falls | 14 (6%) |
| Characteristics . | Value . |
|---|---|
| . | |
| n | 225 |
| Females n (%) | 218 (97%) |
| Age (years) | 63 ± 12 |
| Length of time since diagnosis (years) | 10 ± 12 |
| Blood parameters | |
| Bilirubin | 12 ± 15 |
| ALT | 42 ± 35 |
| ALP | 168 ± 190 |
| Albumin | 41 ± 4.1 |
| Platelets | 250 ± 94 |
| TSH | 2.2 ± 14 |
| IGG | 13 ± 11 |
| Histology | |
| No biopsy (%)/Precirrhotic/cirrhotic | 119 (53%)/ 64 (28%)/42 (19%) |
| Medication | |
| UDCA | 124 (55%) |
| Mean daily dose | 623 mg |
| Potentially culprit medication | 77 (34%) |
| Symptoms described by patient | |
| Loss of consiousness | 16 (7%) |
| Fatigue | 128 (57%) |
| Itch | 71 (31%) |
| Dry eyes | 83 (37%) |
| Dry mouth | 99 (44%) |
| Postural dizziness | 79 (35%) |
| Unexplained Falls | 14 (6%) |
Symptom assessment tool values before and after implementation of the PBC ICP in all patients attending the PBC clinic
| Symptom assessment tool . | Initial assessment . | Repeat assessment . | P . | |||
|---|---|---|---|---|---|---|
| . | RIP group . | Responders . | Non-responders . | Total group . | . | . |
| . | ||||||
| PBC-40 other Symptoms domain | 17 ± 5 | 18 ± 5 | 18 ± 6 | 18 ± 5 | 16 ± 6 | <0.0001 |
| PBC-40 Itch domain | 5 ± 4 | 5 ± 4 | 5 ± 4 | 5 ± 4 | 4 ± 4 | 0.12 |
| PBC-40 Fatigue domain | 26 ± 16 | 32 ± 12 | 33 ± 14 | 32 ± 12 | 31 ± 11 | 0.6 |
| PBC-40 Cognitive domain | 10 ± 6 | 15 ± 7 | 15 ± 7 | 15 ± 7 | 14 ± 7 | 0.05 |
| PBC-40 Social & Emotional domain | 32 ± 14 | 34 ± 12 | 36 ± 12 | 34 ± 12 | 32 ± 13 | 0.15 |
| ESS | 8.7 ± 7.0 | 8.6 ± 5.6 | 9.5 ± 6.1 | 8.9 ± 6.0 | 8.5 ± 5.5 | 0.4 |
| OGS | 3.4 ± 3.6 | 3.5 ± 3.8 | 3.1 ± 3.1 | 3.4 ± 3.6 | 3.6 ± 4.0 | 0.7 |
| Symptom assessment tool . | Initial assessment . | Repeat assessment . | P . | |||
|---|---|---|---|---|---|---|
| . | RIP group . | Responders . | Non-responders . | Total group . | . | . |
| . | ||||||
| PBC-40 other Symptoms domain | 17 ± 5 | 18 ± 5 | 18 ± 6 | 18 ± 5 | 16 ± 6 | <0.0001 |
| PBC-40 Itch domain | 5 ± 4 | 5 ± 4 | 5 ± 4 | 5 ± 4 | 4 ± 4 | 0.12 |
| PBC-40 Fatigue domain | 26 ± 16 | 32 ± 12 | 33 ± 14 | 32 ± 12 | 31 ± 11 | 0.6 |
| PBC-40 Cognitive domain | 10 ± 6 | 15 ± 7 | 15 ± 7 | 15 ± 7 | 14 ± 7 | 0.05 |
| PBC-40 Social & Emotional domain | 32 ± 14 | 34 ± 12 | 36 ± 12 | 34 ± 12 | 32 ± 13 | 0.15 |
| ESS | 8.7 ± 7.0 | 8.6 ± 5.6 | 9.5 ± 6.1 | 8.9 ± 6.0 | 8.5 ± 5.5 | 0.4 |
| OGS | 3.4 ± 3.6 | 3.5 ± 3.8 | 3.1 ± 3.1 | 3.4 ± 3.6 | 3.6 ± 4.0 | 0.7 |
n = 149. All values are shown as mean ± SD, and statistically significant differences are shown in bold. Values are presented for the group who died during follow up (RIP) those who responded to the follow up questionnaires (responders) and those who did not reply to the follow up questionnaires (non-responders).
Symptom assessment tool values before and after implementation of the PBC ICP in all patients attending the PBC clinic
| Symptom assessment tool . | Initial assessment . | Repeat assessment . | P . | |||
|---|---|---|---|---|---|---|
| . | RIP group . | Responders . | Non-responders . | Total group . | . | . |
| . | ||||||
| PBC-40 other Symptoms domain | 17 ± 5 | 18 ± 5 | 18 ± 6 | 18 ± 5 | 16 ± 6 | <0.0001 |
| PBC-40 Itch domain | 5 ± 4 | 5 ± 4 | 5 ± 4 | 5 ± 4 | 4 ± 4 | 0.12 |
| PBC-40 Fatigue domain | 26 ± 16 | 32 ± 12 | 33 ± 14 | 32 ± 12 | 31 ± 11 | 0.6 |
| PBC-40 Cognitive domain | 10 ± 6 | 15 ± 7 | 15 ± 7 | 15 ± 7 | 14 ± 7 | 0.05 |
| PBC-40 Social & Emotional domain | 32 ± 14 | 34 ± 12 | 36 ± 12 | 34 ± 12 | 32 ± 13 | 0.15 |
| ESS | 8.7 ± 7.0 | 8.6 ± 5.6 | 9.5 ± 6.1 | 8.9 ± 6.0 | 8.5 ± 5.5 | 0.4 |
| OGS | 3.4 ± 3.6 | 3.5 ± 3.8 | 3.1 ± 3.1 | 3.4 ± 3.6 | 3.6 ± 4.0 | 0.7 |
| Symptom assessment tool . | Initial assessment . | Repeat assessment . | P . | |||
|---|---|---|---|---|---|---|
| . | RIP group . | Responders . | Non-responders . | Total group . | . | . |
| . | ||||||
| PBC-40 other Symptoms domain | 17 ± 5 | 18 ± 5 | 18 ± 6 | 18 ± 5 | 16 ± 6 | <0.0001 |
| PBC-40 Itch domain | 5 ± 4 | 5 ± 4 | 5 ± 4 | 5 ± 4 | 4 ± 4 | 0.12 |
| PBC-40 Fatigue domain | 26 ± 16 | 32 ± 12 | 33 ± 14 | 32 ± 12 | 31 ± 11 | 0.6 |
| PBC-40 Cognitive domain | 10 ± 6 | 15 ± 7 | 15 ± 7 | 15 ± 7 | 14 ± 7 | 0.05 |
| PBC-40 Social & Emotional domain | 32 ± 14 | 34 ± 12 | 36 ± 12 | 34 ± 12 | 32 ± 13 | 0.15 |
| ESS | 8.7 ± 7.0 | 8.6 ± 5.6 | 9.5 ± 6.1 | 8.9 ± 6.0 | 8.5 ± 5.5 | 0.4 |
| OGS | 3.4 ± 3.6 | 3.5 ± 3.8 | 3.1 ± 3.1 | 3.4 ± 3.6 | 3.6 ± 4.0 | 0.7 |
n = 149. All values are shown as mean ± SD, and statistically significant differences are shown in bold. Values are presented for the group who died during follow up (RIP) those who responded to the follow up questionnaires (responders) and those who did not reply to the follow up questionnaires (non-responders).
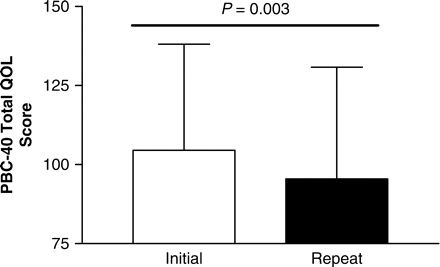
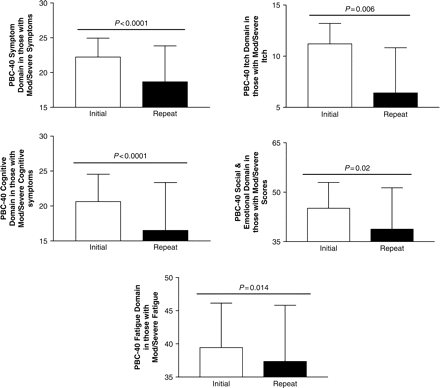
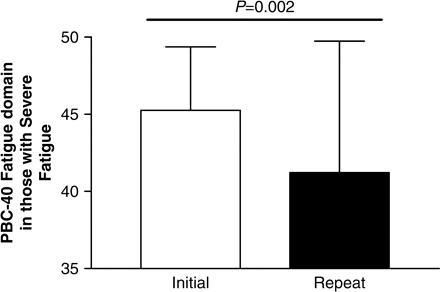
Cumulative symptom burden assessed using the combined PBC-40 domain scores was significantly lower at the follow-up assessment (after implementation of the PBC ICP) compared to initial assessment (before implementation) (Figure 3). All symptom domains improved after implementation of the ICP (Table 2), with the exception of the OGS, with the other Symptom domain and Cognitive domain scores showing significant improvements. When considering subjects with clinically significant symptoms in each symptom domain (defined as moderate and severe symptoms as described before), significant improvements were seen in all PBC-40 symptom domain scores (Figure 4). When those with severe fatigue were examined (n = 38), the improvements in symptomatology were even more dramatic (Figure 5). Considering the recent observation that PBC is associated with excessive daytime sleepiness we also considered those in our clinic cohort who had an ESS consistent with excessive daytime sleepiness (ESS ⩾10). In this group (n = 61), ESS improved from 15 ± 4 to 12 ± 5 (P = 0.002). During the year of follow-up 25 PBC patients with excessive daytime sleepiness were treated with the stimulant modafinil. In contrast change in OGS scores after implementation of our ICP did not reach significance with mean scores in those with orthostatic intolerance (OGS ⩾ 4) improving from 6.8 ± 2.7 to 6.4 ± 4.0 (P = ns).
Significant improvements are seen in cumulative PBC-40 score after implementation of the PBC ICP.
Significant improvements are seen in all domains of the PBC-40 in PBC patients who have moderate/severe symptoms when seen prior to implementation of the PBC ICP.
Significant improvements are seen in PBC-40 fatigue domain scores in PBC patients with severe symptoms when seen prior to implementation of the PBC ICP.
Impact of implementation of the PBC ICP upon investigations and appointments
In addition to improvements in patients QOL implementation of the PBC ICP led to a 14% reduction in review appointments, which allowed a doubling in available new patient slots per clinic. The number of liver function tests performed was reduced by 12%, however the number of abdominal ultrasound tests increased by 30%.
Discussion
Implementation of an ICP that delivers evidence-based care to patients with the chronic liver disease PBC leads to improvements in QOL and reduced review appointments coupled with high levels of patient satisfaction.
The process of development of the PBC ICP within our unit has increased local awareness of this chronic disease. We have improved our service by ensuring we have multidisciplinary documentation which we have confirmed leads to improvements in QOL in our patient group and our experience suggests results in enhanced/improved communication within and out with the unit. Furthermore, the development process has lead to a re-evaluation and clarification of roles and responsibilities within our team, highlighted bottlenecks, reduced unnecessary investigations, duplication and consultations.
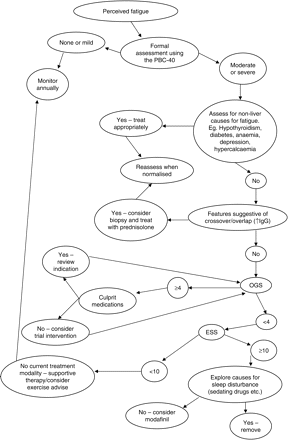
The improvements in ESS scores seen in this study would be consistent with the use in our clinic of a specific management pathway for the treatment of daytime sleepiness that includes addressing sleep hygiene, referral where appropriate to the sleep clinic and the use where appropriate of the stimulant modafinil18,20,21 (Figure 2). Currently, such pathways of care are less established for the management of fatigue associated with autonomic dysfunction, despite our recent observation of the association between autonomic dysfunction and fatigue in PBC.11,16,22,23 This may in part explain the fact that significant improvements in OGS scores were not seen. Further work to develop interventions appropriate for the management of autonomic dysfunction in PBC is needed.
Evaluation of our entire clinic cohort has highlighted potential issues when interpreting QOL studies in groups of patients recruited from hospital-based clinics. Compared to our previously published geographically defined cohort of patients,16 the clinic-based cohort is older, and more symptomatic overall, with more severe liver disease. We would suggest that this population sampling may go someway to explaining the differences in fatigue and symptom prevalence in the PBC literature.
In keeping with the Clinical Governance agenda, the ICP ensures standardization and consistency of care for all patients, allows continuous quality improvement, eases audit and evaluation of outcomes and ensures adherence in all patients to the evidence from recent clinical studies by integrating latest evidence-based practice and guidelines within the ICP. It is gratifying to show that the use of such a structured approach to symptomatology in a specialist PBC clinic leads to improvements in QOL in this chronic disease. It is possible that some other (undefined) intervention led to the improvements seen in our patient QOL, however, no specific changes were made in our clinic structure or our patient management other than implementation of the pathway so it is difficult to determine what other interventions might have been responsible for the improvements seen.
Our findings have major implications for other chronic disease settings where investment in a similar approach has the real potential to lead to significant benefits for patients. Development of an ICP is time-consuming and requires a willingness of all staff within a clinical area to drive the project forward and to sign up wholeheartedly to its success. The development process involves resource implications and a willingness of staff in management to assist the development and implementation process. But we would argue that this study highlights the potential clinical and financial benefits that may result. Our ICP allows the delivery of evidence-based investigation and intervention within a multidisciplinary framework and could be adopted by new and existing dedicated PBC services, gastroenterology and hepatology services. Furthermore, the application of a similar structured approach to other chronic diseases has potential benefits that extend beyond liver disease.
It is now our intention that we move towards an electronic ICP to be completed directly in the clinic onto a personal computer. This will then generate the clinical record that will form an integral part of the patient's medical notes and allow us to move rapidly when the electronic patient record is implemented.
Though copyrighted the ICP will be published on the National Electronic Library for Health (NLH) website (http://www.library.nhs.uk/pathways/ViewResource.aspx?resID=270519&tabID=288).
The authors are more than willing to share their experiences of this developmental process with any interested parties.
Conflict of interest: None declared.